Multiparameter autoantibody testing in autoimmune neurological diseases
In recent years, there has been a growing understanding of the pathology of autoimmune neurological disorders, and the identification of the causal autoantibodies has allowed the definition of new diseases. As these diseases have a multifaceted clinical presentation, comprehensive neural autoantibody testing is a central part of diagnosis. This article describe the current knowledge of is increasingly diagnosed group of diseases.
by Dr Jacqueline Gosink
Overview
Neurological disorders such as memory deficits, behavioural abnormalities, movement disorders, seizures and psychosis can be caused by underlying autoimmune reactions. In many instances there is a connection with a malignant disease. The spectrum of diagnostically relevant autoantibodies in neurological syndromes has grown rapidly in recent years due to the identification of many novel target antigens. The autoantibodies are directed against diverse neuronal intracellular antigens, receptors, ion channels, synaptic antigens, as well as proteins of the neuromuscular junction. In unexplained neurological cases, a comprehensive autoantibody analysis can help to secure a diagnosis and in some cases may also provide the first indication of a tumour. Since some autoantibodies occur only rarely and clinical symptoms may overlap, multiparameter testing is recommended over selective or sequential analyses to avoid diagnostic gaps and establish a diagnosis as rapidly as possible.
Paraneoplastic neurologic syndromes
Paraneoplastic neurologic syndromes (PNS) are immune-mediated disorders of the central and peripheral nervous system caused by the remote effects of malignant tumours. They develop in around 1 in 300 patients with cancer, for example small-cell lung carcinoma, breast cancer, ovarian cancer, testicular cancer, non-small-cell lung carcinoma, thymoma or lymphomas. Clinical manifestations of PNS include encephalomyelitis, limbic encephalitis, rapidly progressing cerebellar syndrome, opsoclonus-myoclonus, sensory neuropathy, gastrointestinal pseudo-obstruction and Lambert–Eaton myasthenic syndrome. New classification criteria have recently been proposed to reflect advances in the understanding of these diseases [1]. The PNS-Care Score takes into account clinical phenotype, detected antibodies and presence/absence of cancer, including at follow-up, to yield a classification level of definite, probable, possible or non- PNS. High-risk phenotypes, previously classified as classical PNS, are strongly linked to an underlying cancer, and autoantibodies serve as important biomarkers in these diseases. High-risk antibodies are connected to cancer in 70% to over 90% of cases depending on the antibody, and a positive result for these antibodies should always be followed by a tumour search directed by the clinical phenotype and the antibody specificity. If the initial tumour screening is negative, it should be repeated every four to six months over a time period of two years. The high-risk antibodies in PNS predominantly target intracellular antigens, and include Hu, Ri, Yo, amphiphysin, CV2, SOX1, Ma/Ta, recoverin, Zic4, titin, ITPR1 and CARP. Anti-DNER/Tr antibodies target a receptor in the Purkinje cells of the cerebellum and are connected to Hodgkin lymphoma.
Autoimmune encephalitides
Autoimmune encephalitides are a group of diseases characterized by rapidly progressing encephalopathy [2] and may occur with or without cancer (intermediate-risk phenotypes). They manifest with a range of symptoms including seizures and neuropsychiatric disorders. These diseases generally respond well to immunotherapy, but treatment must be started early to prevent irreversible damage to the brain. Recently, many novel autoantibody targets have been identified and, based on this, new disease subtypes with a variety of clinical manifestations defined. The autoantibodies are directed against neuronal cell-surface and synaptic antigens and there is evidence that they play a direct pathogenic role. These antibodies have a moderate or low association with cancer and are hence classified as intermediate-risk (30 to 70%) or low-risk antibodies (<30%) for PNS. In laboratory practice autoantibodies against neuronal surface targets are found three times more frequently than those against intracellular antigens. The most frequent and best characterized disease type is anti-Nmethyl- D-aspartate (NMDA) receptor encephalitis [3, 4]. The target antigen is the membrane-spanning channel subunit GluN1 of the NMDA receptor. Symptoms of anti-NMDA receptor encephalitis encompass psychosis, movement disorders, seizures, dysautonomia and coma. The disease is most common in young adults, with a high female to male ratio, and approximately 40–50% of patients present with a neoplasm, predominantly ovarian teratoma. Herpes simplex virus-1 encephalitis has recently been found to be among the triggers of anti-NMDA receptor encephalitis. Other forms of autoimmune encephalitis are linked to autoantibodies against further cell-surface antigens [4, 5]. LGI1 and CASPR2 are specific target antigens of autoantibodies formerly thought to be directed against voltage-gated potassium channels. Anti-LGI1 reactivity is tightly associated with limbic encephalitis and a tumour frequency of 5 to 10% of cases. Anti-CASPR2 autoantibodies have been described in patients with mostly encephalitis and/or peripheral nerve dysfunction (Morvan’s syndrome). Twenty to fifty percent of cases are linked to thymoma. Anti-DPPX encephalitis is a multifocal neurological disorder with prominent hyperexcitability of the central nervous system (CNS) and rare (<10%) association with lymphoma. Autoimmune encephalitis with reactivity against GABAB receptors is characterized by very prominent seizures, memory loss and confusion. Neoplasms, especially small-cell lung carcinoma, occur in about half of patients. Patients with anti-AMPA receptor encephalitis commonly exhibit subacute confusion, memory deficits, seizures and sometimes dementia. Seventy percent of cases are paraneoplastic, affecting the lungs, thymus and breast. IgLON5 autoantibodies are associated with parasomnia and tauopathy and do not have a known tumour association. Stiff-person syndrome
Stiff-person syndrome (SPS) is a rare disease of the CNS which manifests with progressive muscle stiffness, as well as spontaneous or triggered spasms. Up to 80% of patients exhibit anti-glutamic acid decarboxylase (GAD) antibodies. Around 5% of SPS cases are paraneoplastic and usually associated with antibodies against amphiphysin.
Neuromyelitis optica spectrum diseases
Neuromyelitis optica spectrum diseases (NMOSD) are acquired demyelinating disorders of the CNS and are characterized by degradation of the insulating sheath of the optical nerves and the spinal cord [7]. Symptoms encompass acute visual disorders including blindness, impaired mobility and loss of bladder and bowel control. NMOSD need be distinguished from the more frequent demyelinating CNS disease multiple sclerosis (MS) as therapy differs.
NMOSD are associated with highly specific pathogenic autoantibodies targeting the CNS water-channel protein aquaporin-4 (AQP-4), which are detected in 60 to 90% of cases. Detection of anti-AQP-4 can secure a diagnosis of NMOSD and enables serological differentiation from MS. Autoantibodies against myelin oligodendrocyte glycoprotein (MOG) occur in around 20% of anti-AQP-4-negative NMOSD cases, as well as in other demyelinating diseases of the CNS, and their determination provides an additional aid for delimitation from classic MS.
Myasthenia syndrome
In myasthenia gravis (MG) the dominant symptom is muscle weakness caused by antibody-mediated transmission disorders of the neuromuscular synapses. Antibodies against nicotinic acetylcholine receptor (AChR) are pathogenic and detected in around 85% of MG patients [8]. Ten to fifteen percent of MG patients develop thymoma, and almost all of these cases are AChR antibody positive. Around 6% of MG patients are AChR antibody negative but positive for antibodies against muscle-specific kinase (MuSK), which are associated with a generalized severe form of MG. Parallel determination of both types of antibody can increase the serological detection rate for MG.
Autoimmune neuropathies
The peripheral nervous system can also be the target of autoaggression, affecting nerves, ganglia or myelin sheaths. Autoantibodies against gangliosides are characteristic markers for Guillain- Barré syndrome (GBS) and its variants, for example acute motor axonal neuropathy, Miller Fisher syndrome and multifocal motor neuropathy. Around two thirds of cases of GBS are triggered by an infection, mostly gastrointestinal or respiratory illnesses.
Autoantibody diagnostics in neurological diseases
Autoantibody analysis is an important component of the diagnostic work-up in neurological diseases, especially in cases where no other cause, such as viral encephalitis, can be identified. Since autoimmune neurological diseases have overlapping syndromes and many of the associated autoantibodies occur only rarely, multiparameter antibody screening is recommended. Analysis of both serum and cerebrospinal fluid provides the greatest detection rate, as the specificity and sensitivity of the detection can vary between these sample materials depending on the antibody present. Only IgG antibodies should be taken into account in the analysis. Test methods for autoantibodies in neurological diseases include multiplex indirect immunofluorescence tests based on tissue substrates and recombinant antigen-expressing cells, multiparameter immunoblot assays, enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA).
Multiplex indirect immunofluorescence
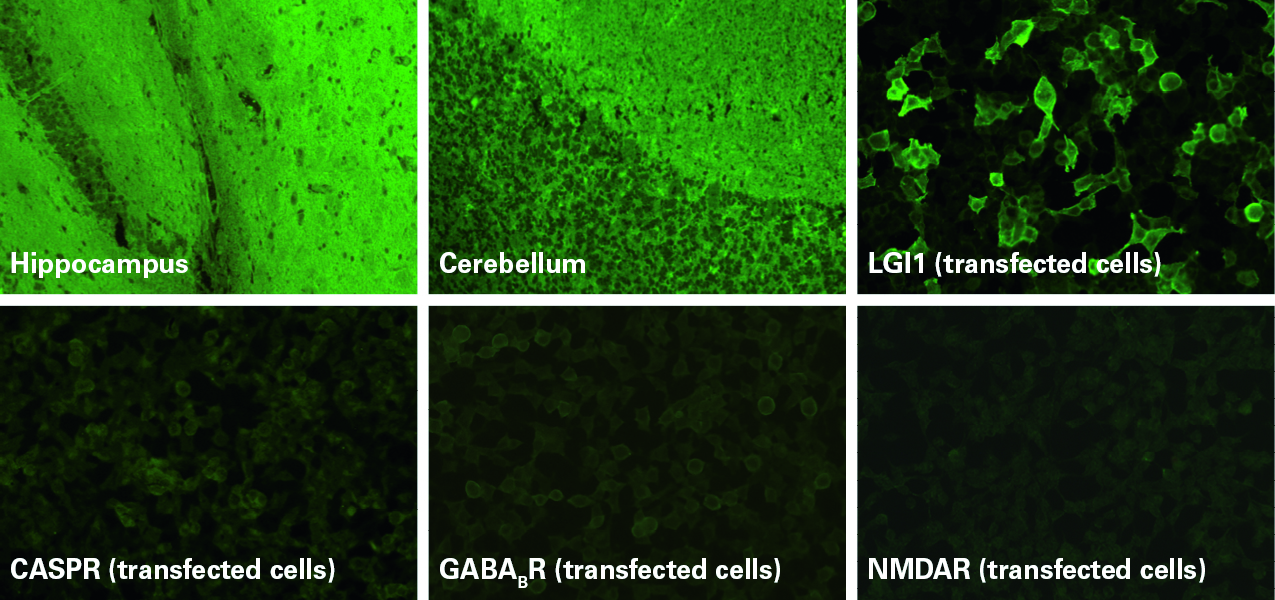
Indirect immunofluorescence using sections of tissues such as nerves, cerebellum, hippocampus and intestine provides a broad autoantibody screening and also enables detection of autoantibodies against as yet unidentified target antigens.
Transfected-cell substrates allow monospecific detection of autoantibodies targeting defined antigens (Fig. 1). The recombinant cells heterologously express the antigens on their surface, generally at a higher concentration per cell than in the native tissue, which allows a more sensitive detection of the corresponding autoantibodies. To achieve maximal diagnostic performance, only the relevant epitopes of the autoantigens are expressed. These tests are particular useful for antigens that are conformation-dependent and fragile and therefore not suited to solid-phase detection methods, such as ELISA or immunoblot. Many neuronal cell-surface antigens fall into this category. Recombinant-cell assays also offer the advantage that they can be developed quickly, enabling rapid introduction of novel parameters into the diagnostic routine.
Recombinant-cell substrates are now available for a wide variety of target antigens, including NMDA receptors, AMPA 1/2 receptors, GABAB receptors, LGI1, CASPR2, DPPX, IgLON5, GAD65, Zic4, DNER/Tr, AQP-4, MOG, AChR (adult/fetal) and MuSK. With BIOCHIP technology, different substrates can be variably combined in one reaction field and incubated in parallel for a comprehensive autoantibody analysis. Different mosaics tailored to different diagnostic applications have been developed, for example the Autoimmune Encephalitis Mosaic 6 containing the six substrates NMDAR, CASPR2, AMAR1/2, LGI1, DPPX and GABAB1/B2, the Myasthenia gravis Mosaic for simultaneous determination of AChR and MuSK antibodies, and the NMOSD Screen 1 for parallel analysis of AQP-4 and MOG antibodies. Results from recombinant-cell substrates can now also be evaluated automatically using EUROPattern automated microscope systems, providing increased standardization and efficiency in the autoantibody analysis.
ELISA/RIA
Anti-acetylcholine receptor antibodies are optimally determined using an antigen mix of adult and fetal acetylcholine receptors. This antigen mix is used in the Anti-Acetylcholine Receptor ELISA and RIA from EUROIMMUN and ensures highly sensitive and specific detection of these antibodies.
Perspectives
The diagnosis and treatment of autoimmune neurological diseases has undergone a paradigm shift in recent years owing to the identification of a plethora of associated autoantibodies and the definition of new disease entities with multifaceted clinical presentation. Autoimmune encephalitis, in particular, has gone from being rarely diagnosed 20 years ago to having a prevalence and incidence comparable to that of infectious encephalitis, with an increasing tendency. Antibodymediated neurological disorders, especially NMDA receptor encephalitis, also serve as models for research into the pathogenic mechanisms of autoantibodies in neurological and psychiatric syndromes. It is hoped that a better understanding of the molecular mechanisms behind the immunological dysfunction could lead to more tailored immunotherapy options. In the proposed new PNS diagnostic criteria, comprehensive neural autoantibody testing represents a central pillar and together with the clinical phenotype serves to hone the tumour screening. The spectrum of autoantibodies in neurological diseases will likely continue to grow in the coming future. New discoveries will enrich the diagnostic yield further by expanding the panel of testable parameters. This will help to close the remaining diagnostic gaps and enable more patients to benefit from life-saving therapy.
The author
Jacqueline Gosink PhD EUROIMMUN Medizinische Labordiagnostika
AG, 23560 Lubeck, Germany
E-mail: j.gosink@euroimmun.de
References
1. Graus F, Vogrig A, Muñiz-Castrillo S, Antoine JG, Desestret V, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm 2021; 8(4): e1014 (https://nn.neurology.org/content/8/4/e1014.long).
2. Abboud H, Probasco JC, Irani S, Ances B, Benavides DR, et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry 2021; 92(7): 757–768 (https://jnnp.bmj.com/content/92/7/757.long).
3. Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol 2019; 18(11): 1045–1057.
4. Prüss H. Autoantibodies in neurological disease. Nature Reviews Immunol 2021; 1–16 (https://www.nature.com/articles/s41577-021-00543-w).
5 Leypoldt F, Armangué T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci 2015; 1338(1): 94–114 (https://bit.ly/3H0PLFy).
6. Dalmau J. NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: The 2016 Cotzias Lecture. Neurology 2016; 87(23): 2471–2482 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5177671/).
7. Prasad S, Chen J. What you need to know about AQP4, MOG, and NMOSD. Semin Neurol 2019; 39(6): 718–731.
8. Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. 2020; 11: 212 (https://www.frontiersin.org/articles/10.3389/fimmu.2020.00212/full).