New developments in the early diagnosis of ovarian cancer
Ovarian cancer is difficult to diagnose early, with consequent poor survival. Evidence suggests many cases may originate in precursor lesions in the fallopian tubes. Differential expression of specific proteins in the fallopian tubes of women with high-grade serous ovarian cancer, detected by immunohistochemistry, shows promise as a potential novel diagnostic marker.
by Dr Kezia Gaitskell and Prof. Ahmed Ashour Ahmed
Background
Ovarian cancer is the seventh greatest cause of cancer mortality amongst women worldwide, and the fifth greatest cause amongst women in more developed regions [1]. In the USA, 60% of women with ovarian cancer already have distant metastases at diagnosis, for which the 5-year survival is less than 30% [2]. Early clinical diagnosis of ovarian cancer is difficult, as symptoms are often non-specific, such as abdominal distention, urinary frequency, or abdominal pain [3].
Current evidence on ovarian cancer diagnosis and screening
Diagnostic investigations include imaging (e.g. ultrasound, CT or MRI of the pelvis and abdomen), together with blood tests for tumour markers – particularly cancer antigen 125 (CA-125) [4]. However, although CA-125 is the main biomarker used in the diagnosis of ovarian cancer, it is far from perfect in sensitivity and specificity: although approximately 80% of women with epithelial ovarian cancer will have a CA-125 concentration above the cut-off value of 35 IU/mL, CA-125 may also be elevated with other cancers (including liver, pancreatic, lung, and endometrial cancers), and physiological or benign conditions (including menses, pregnancy, cirrhosis, salpingitis, pancreatitis and endometriosis) [5].
A variety of additional putative tumour markers have been suggested for use in combination with CA-125, but current guidelines from the National Comprehensive Cancer Network in the USA are that there is insufficient evidence for their usefulness in detecting early-stage ovarian cancer [4], although the European Group on Tumor Markers suggests that human epididymis protein 4 (HE4) may be helpful for the differential diagnosis of pelvic masses, particularly in premenopausal women [5].
There has been considerable interest in finding markers of early disease, that could enable earlier diagnosis or screening for ovarian cancer. Two large randomized controlled trials have been performed of screening for ovarian cancer: the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) in the USA [6], and the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) in the UK [7]. Both trials randomized women to either no screening, or screening with the CA-125 blood test with or without trans-vaginal ultrasound. Unfortunately, neither trial could demonstrate a clear mortality benefit with screening, although there was a suggestion of benefit in some secondary analyses in the UKCTOCS trial [7].
New hypotheses of the origins of ovarian cancer
The search for potential early markers of ovarian cancer is also affected by the increasing evidence of heterogeneity between the tumour subtypes. Ovarian cancer has traditionally been divided into subtypes on the basis of microscopic morphology, the most common types being serous, endometrioid, clear cell, and mucinous tumours. There is growing evidence that these different histological tumour subtypes have different characteristic genetic mutations, and may have distinct origins [8, 9]. In particular, there is evidence that many cases of high-grade serous ovarian cancer (the most common subtype) may arise from precursor lesions in the fallopian tube epithelium, known as serous tubal intraepithelial carcinoma (STIC) (reviewed by Nik et al. [10]). These STIC lesions show dysplastic morphological changes, and also tend to show the mutations in the tumour-suppressor gene TP53 that are characteristic of high-grade serous ovarian carcinoma, and increased expression of the proliferation marker Ki-67. There is also evidence that some cases of endometrioid and clear cell ovarian cancer (less common subtypes) may arise from endometriosis (reviewed by Munksgaard & Blaakaer [11]). The origins of low-grade serous tumours and mucinous carcinomas are less certain, although several hypotheses exist.
The hypothesis that many, if not most, high-grade serous ‘ovarian’ cancers may in fact arise from the fallopian tubes has led to increasing interest in exploring changes in the fallopian tubes as potential early markers. The discovery of STIC lesions is interesting in terms of improving our understanding of pathogenesis, but is not currently useful for identifying changes early in malignancy, or pre-malignancy, in clinical practice. One limitation is that STIC lesions tend to be very focal, and are most common at the fimbrial end of the fallopian tubes, adjacent to the ovary, which is difficult to access without surgical removal of the fallopian tubes.
New findings regarding the role of SOX2
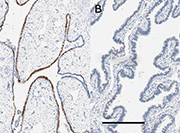
We investigated increased expression of SOX2, a key stem cell differentiation gene, as a possible marker of high-grade serous carcinogenesis within the fallopian tubes. We chose SOX2 because work from our group had shown that mutations at several sites near the SOX2 gene were ubiquitous in samples of ovarian cancer taken from multiple locations and time points in a single patient, indicating that they acted as early ‘driver’ mutations [12]. We showed that SOX2 expression (detected using immunohistochemistry) was significantly increased in the fallopian tube epithelial cells of women with high-grade serous ovarian cancer, compared to women with endometrial cancer or benign disease (e.g. uterine fibroids) [12], as illustrated in Figure 1.
We also found that SOX2 expression in the fallopian tubes was significantly increased in women with germline mutations in the tumour suppressor genes BRCA1 and BRCA2, who are known to be at higher risk of breast and ovarian cancer [12]. These women with BRCA1/2 mutations had their ovaries and fallopian tubes removed to reduce their subsequent risk of cancer, but did not have evident cancer at the time of surgery. Thus, the finding that elevated SOX2 expression was detectable in their fallopian tubes suggests that increased SOX2 expression may be an early sign of precancerous changes within the fallopian tubes.
Potential future implications
Our observation that SOX2 expression is increased in the fallopian tube epithelial cells of women with high-grade serous ovarian cancer, and women with BRCA1/2 mutations, compared to women with other cancers or benign disease, suggests that SOX2 might have a potential role as a biomarker in the early diagnosis of ovarian cancer. However, several challenges remain before testing for SOX2 expression could be considered in clinical practice – particularly the anatomical difficulty of sampling the fallopian tube epithelium without invasive surgery, and the fact that SOX2 is a nuclear marker. Our research group is currently exploring other potential cell-surface markers that correlate with SOX2 expression, which might be easier to detect.
Summary
There are many challenges in the early diagnosis of ovarian cancer. New evidence of the possible tubal origins of high-grade serous ovarian cancer is changing the approach to identifying potential new biomarkers of early disease. SOX2 has emerged as a promising marker, but further work is needed before it would be suitable for routine clinical practice.
References
1. Ferlay J, Soerjomataram I, et al. GLOBOCAN 2012 v1.0: Estimated cancer incidence and mortality worldwide in 2012. International Agency for Research on Cancer/World Health Organization 2013 (http://globocan.iarc.fr/Default.aspx).
2. Howlader N, Noone AM, et al. Cronin KA (eds). SEER Cancer Statistics Review, 1975-2013. National Cancer Institute, Bethesda, MD, USA 2016 (http://seer.cancer.gov/csr/1975_2013/).
3. Hamilton W, Peters TJ, et al. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ 2009; 339: b2998.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, Version 1.2016. Ft. Washington, PA, USA. National Comprehensive Cancer Network 2016 (https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf).
5. Soletormos G, Duffy MJ, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European group on tumor markers. Int J Gynecol Cancer 2016; 26(1): 43–51.
6. Buys SS, Partridge E, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305(22): 2295–2303.
7. Jacobs IJ, Menon U, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016; 387(10022): 945–956.
8. Kurman RJ, Shih IeM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum Pathol. 2011; 42(7): 918–931.
9. Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012; 460(3): 237–249.
10. Nik NN, Vang R, et al. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu Rev Pathol. 2014; 9: 27–45.
11. Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol. 2012; 124(1): 164–169.
12. Hellner K, Miranda F, et al. Premalignant SOX2 overexpression in the fallopian tubes of ovarian cancer patients: discovery and validation studies. EBioMedicine 2016; 10: 137–149.
The authors
Kezia Gaitskell*1,2 BM BCh; Ahmed Ashour Ahmed1,2 MBBCh, MRCOG, PhD
1Ovarian Cancer Cell Laboratory, Weatherall Institute of Molecular Medicine, University of Oxford, Headington, Oxford OX3 9DS, UK
2Nuffield Department of Obstetrics and Gynaecology, University of Oxford, Women’s Centre, John Radcliffe Hospital, Oxford OX3 9DU, UK
*Corresponding author
E-mail: Kezia.gaitskell@ceu.ox.ac.uk