New psychoactive substances pose a challenge for drug testing laboratories
New psychoactive substances (NPS) reach the recreational drugs market at a fast pace and are of concern because of potential health risks. In addition to not being legally regulated, NPS escape detection in standard drug tests. Drug testing laboratories, therefore, must adapt their analytical methods to also cover these new substances. For screening and confirmation of NPS, mass-spectrometric multicomponent methods are useful.
by Prof. Olof Beck and Prof. Anders Helander
New psychoactive substances
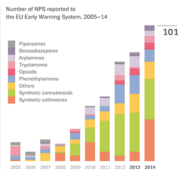
The emergence of new drugs of abuse that are designed to circumvent narcotics legislation by slight chemical structural modifications of already classified drugs represents an ever increasing problem [1, 2]. Nowadays, this phenomenon is commonly termed ‘new psychoactive substances’ or ‘NPS’, but also other names such as designer drugs, legal highs, research chemicals, smart drugs, bath salts, and spice have been and are used. The NPS problem is of global concern but may vary in extent between countries, partly due to national differences in legislation and drug culture. Statistics from the EU Early Warning System operated by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) and Europol on the number of NPS reported for the first time in Europe on a yearly basis gives a good insight on the progress of this phenomenon (Fig. 1) [2]. Over the past 6 years particularly, it has escalated to the level of more than 100 new substances in 2014 (i.e. about two new substances each week on average). The NPS market was long dominated by stimulants and synthetic cannabinoids but currently comprises all classes of abused substances [2].
Problems related to NPS
NPS are of particular concern because they can be sold openly in web-based shops and elsewhere and thereby reach new drug users that are attracted by their ‘legal’ status. Of public concern are the unforeseen toxic effects of NPS, as using these uncontrolled and unsafe substances and products may lead to severe intoxication and even death [1, 3]. In Sweden, the progress of the NPS phenomenon and associated harmful effects has been followed in a collaborative project between the Department of Laboratory Medicine at the Karolinska University Hospital and the Karolinska Institutet, and the Swedish Poisons Information Center [3, 4]. This project, named STRIDA, enrolls patients with suspected NPS intoxication presenting in emergency departments all over the country. By combining the results from laboratory investigations of serum and urine samples with clinical information, new knowledge about NPS prevalence and toxicity is compiled. Since the start in 2010, the STRIDA project has documented over 2000 non-fatal but often severe acute intoxication cases involving a large number of different NPS. Polydrug use is commonly seen in these cases [3].
NPS in drug screening
One reason for using NPS instead of conventional drugs of abuse may be that NPS often remain undetected in standard drug testing procedures. Accordingly they are especially attractive alternatives for individuals who want to minimize the risk of being detected, such as in workplace drug testing and drug rehabilitation programmes.
The established procedure for drug testing is to use initial screening by immunoassays and then to confirm positive samples using methods based on the more sensitive and selective mass spectrometry (MS) technique. On one hand, the NPS present a challenge for the immunoassay screening, as available methods are typically directed only towards the conventional substances, e.g. amphetamines (amphetamine and methamphetamine), tetrahydrocannabinolcarboxylic acid (THC, cannabis), morphine (heroin), and benzoyl ecgonine (cocaine). On the other hand, as NPS are often designed to mimic and are chemical derivatives of conventional drugs, there is a possibility that certain NPS will also bind to (i.e. cross-react with) the antibodies used in immunoassay screening methods. And this is indeed the case. However, when these ‘false-positive’ screening results are subjected to confirmatory analysis by methods based on MS detection, they will turn out negative (i.e. ‘false-negative’ for drug use), if the MS method is only directed toward the standard set of abused drugs.
Cross-reactivity of NPS in immunoassays
When ecstasy (3,4-methylenedioxymethamphetamine, MDMA) became established as a street drug, interest emerged to detect it in immunoassay screening. MDMA and its metabolite 3,4-methylenedioxyamphetamine (MDA) were found to be detectable in existing assays for amphetamine and methamphetamine, due to a high degree of cross-reactivity for these compounds [5]. Likewise, also other new amphetamine-like substances were detectable [6].
However, although many NPS showed low cross-reactivity in commercial immunoassays [7, 8], the stimulant methylenedioxypyrovalerone (MDPV) was reported to cross-react in the CEDIA phencyclidine test [9]. A study from the authors’ laboratory comprising 45 NPS confirmed that several possessed chemical similarities leading to high cross-reactivity in the immunochemical screening tests commonly employed in routine urine drug testing [10]. The detectability of NPS observed to possess cross-reactivity was further confirmed by analysis of urine specimens from authentic intoxication cases included in the STRIDA project (Table 1). Given a more widespread use of new drugs among individuals subjected to drug testing, an increased number of unconfirmed positive screening results may occur.
The cross-reactivity for NPS in current screening assays may be seen as a problem or as a possibility to detect more substances. One possibility for improved drug testing is to include the most common new substances in the confirmation methods. As ecstasy became established as an illicit drug, new immunochemical screening tests for amphetamine/methamphetamine were developed that also included MDMA and MDA. Authentic case samples were used to demonstrate the capability of several commercial amphetamine class screening tests to detect MDMA/MDA. At that time, cross-reactivity towards the new ‘amphetamine’ analytes was wanted [5]. With the advent of the large number of NPS, both legal and illegal, the strategy to also cover new substances in the screening assays for classical narcotic drug substances may not be feasible. For example, the multitude of new synthetic cannabinoids (‘spice’) have not been incorporated in screening tests for THC, but resulted in the development of new independent tests [11].
One approach put forward to understand the potential of immunoassays to detect NPS is to use molecular similarity models [12]. Interestingly, the work of Petrie and co-workers [13] included such a molecular modelling method to predict the cross-reactivity of 261 amphetamine-like compounds. However, when comparing the theoretical data with our experimental data for one compound, the predicted reactivity for butylone was 10 times lower than that observed. In a more recent publication, it was proposed that molecular similarity models could be used to design new immunoassays with sensitivity for a larger number of target compounds [14].
NPS analysis by mass spectrometry
Another analytical strategy to cover NPS in drug testing is to employ MS-based ‘screening’ methods. As part of the STRIDA project, a multicomponent analytical MS method for NPS analysis in urine and serum specimens has been developed [15]. The method uses MS in combination with liquid chromatography (LC-MS/MS in selected-reaction monitoring mode) and is continuously updated as new NPS appear. There are also other methods for multicomponent screening of drugs in urine and plasma/serum, which proves that this technology can be employed in routine drug testing [16].
The LC-MS/MS technique has great potential for drug testing and for clinical laboratories in general. There are examples of laboratories that have already successfully replaced immunoassay screening by MS methods, also for the conventional drugs of abuse [17]. One way to make this possible and cost-effective is to use simple sample preparation procedures, e.g. a simple dilution of urine with internal standards [16]. When studying the cross-reactivity of 30 NPS in commercial ELISA tests for serum and blood, only a few were found to display cross-reactivity, and it was therefore proposed that MS methods should be used in future drug screening [18]. One attraction of MS-based screening is that accurate results are already obtained from the initial analytical step, which may be especially important in cases of acute intoxication (Fig. 2).
Potential of high-resolution MS
One promising technique for drug screening is high-resolution MS (HRMS) [19]. In the HRMS technique, the acquisition of data can be made with an untargeted design. Thousands of substances can be monitored at the same time without the need for optimizing MS parameters for each compound. In addition, new compounds can be searched for retrospectively.
Conclusion
The NPS present a challenge for drug testing laboratories and calls for novel drug screening strategies. It is likely that the current broader spectrum of abused psychoactive drugs will persist in at least in the foreseeable future. This new drug situation has put the performance of drug testing into focus and indicates that drug testing laboratories will play a more important role, as on-site drug screening using dipsticks is likely to lose significance.
References
1. Lewin AH, Seltzman HH, Carroll FI, Mascarella SW, Reddy PA. Emergence and properties of spice and bath salts: A medicinal chemistry perspective. Life Sci. 2014; 97: 9–19.
2. EMCDDA. New psychoactive substances in Europe. An update from the EU Early Warning System (March 2015). 2015. Available at: http://www.emcdda.europa.eu/attachements.cfm/att_235958_EN_TD0415135ENN.pdf.
3. Helander A, Bäckberg M, Hultén P, Al-Saffar Y, Beck O. Detection of new psychoactive substance use among emergency room patients: results from the Swedish STRIDA project. Forensic Sci Int. 2014; 243: 23–29.
4. Helander A, Bäckberg M, Beck O. MT-45, a new psychoactive substance associated with hearing loss and unconsciousness. Clin Toxicol. 2014; 52(8): 901–904.
5. Hsu J, Liu C, Hsu CP, Tsay WI, Li JH, Lin DL, Liu RH. Performance characteristics of selected immunoassays for preliminary test of 3,4-methylenedioxymethamphetamine, methamphetamine, and related drugs in urine specimens. J Anal Toxicol. 2003; 27: 471–478.
6. Apollonio LG, Whittall IR, Pianca DJ, Kyd JM, Haher WA. Matrix effect and cross-reactivity of select amphetamine-type substances, designer analogues, and putrefactive amines using Bio-Quant direct Elisa presumptive assays for amphetamine and methamphetamine. J Anal Toxicol. 2007; 31: 208–213.
7. Kerrigan S, Mellon MB, Banuelos S, Arndt C. Evaluation of commercial enzyme-linked immuno assays to identify psychedelic phenethylamines. J Anal Toxicol. 2011; 35: 444–451.
8. Bell C, George C, Kicman AT, Traynor A. Development of a rapid LC-MS/MS method for direct urinalysis of designer drugs. Drug Test Anal. 2011; 3: 496–504.
9. Macher AM, Penders TM. False-positive phencyclidine immunoassay results caused by 3,4-methylenedioxypyrovalerone (MDPV). Drug Test Anal. 2012; 5: 130–132.
10. Beck O, Rausberg L, Al-Saffar Y, Villen T, Karlsson L, Hansson T, Helander A. Detectability of new psychoactive substances, ‘legal highs’, in CEDIA, EMIT, and KIMS immunochemical screening assays for drugs of abuse. Drug Test Anal. 2014; 6: 492–499.
11. Arntson A, Ofsa B, Lancaster D, Simon JR, McMullin M, Logan B. Validation of a novel immunoassay for the detection of synthetic cannabinoids and metabolites in urine specimens. J Anal Toxicol. 2013; 37: 284–290.
12. Krasowski MD, Pizon AF, Siam MG, Giannoutsos S, Iyer M, Ekins S. Using molecular similarity to highlight the challenges of routine immunoassay-based drug of abuse/toxicology screening in emergency medicine. BMC Emerg Med. 2009; 9: 5.
13. Petrie M, Lynch KL, Ekins S, Chang JS, Goetz RJ, Wu AHB, Krasowski MD. Cross-reactivity studies and predictive modeling of “Bath Salts” and other amphetamine-type stimulants with amphetamine screening immunoassays. Clin Toxicol. 2013; 51: 83–91.
14. Krasowski MD, Ekins S. Using cheminformatics to predict cross reactivity of “designer drugs” to their currently available immunoassays. J. Cheminform. 2014; 6: 22.
15. Al-Saffar Y, Stephanson NN, Beck O. Multicomponent LC-MS/MS screening method for detection of new psychoactive drugs, legal highs, in urine – experience from the Swedish population. J Chromatogr B 2013; 930: 112–120.
16. Beck O, Ericsson M. Methods for urine drug testing using one-step dilution and direct injection in combination with LC-MS/MS and LC-HRMS. Bioanalysis 2014; 6 : 2229–2244.
17. Eichhorst JC, Etter ML, Rousseaux N, Lehotay DC. Drugs of abuse testing by tandem mass spectrometry: A rapid, simple method to replace immunoassays. Clin Biochem. 2009; 42: 1531–1542.
18. Swortwood MJ, Hearn WL, DeCaprio AP. Cross-reactivity of designer drugs, including cathinone derivatives, in commercial enzyme-linked immunosorbent assays. 2014; 6: 716–727.
19. Maurer HH. What is the future of (ultra) high performance liquid chromatography coupled to low and high resolution mass spectrometry for toxicological drug screening? J Chromatogr A 2013; 1292: 19–24.
The authors
Olof Beck*1,3 PhD and Anders Helander2,3 PhD
1Department of Clinical Pharmacology, Karolinska University Laboratory Huddinge, Sweden
2Department of Clinical Chemistry, Karolinska University Laboratory Huddinge, Sweden
3Department of Laboratory Medicine, Karolinska Institutet, Stockholm, Sweden
*Corresponding author
E-mail: olof.beck@karolinska.se