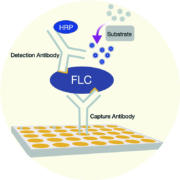
Next generation free light chain assay
Sebia is expanding its offer with two innovative CE-IVD marked assays for the determination of serum free light chains: sebia FLC Kappa and sebia FLC Lambda. Serum free light chain assays (sFLC) form part of the diagnosis, prognosis and monitoring of multiple myeloma and other monoclonal gammopathies.
Sebia FLC Kappa and sebia FLC Lambda are clinically validated for patient diagnosis and follow-up. These tests are not affected by the analytical limits observed on the current nephelometric and turbidimetric methods used, i.e. antigen excess leading to underestimation of the result, small measuring range resulting in many re-dilutions and overestimation of the sFLC value leading to a discrepancy with electrophoresis results.
Overcoming these limitations, sebia FLC provides the added benefits of a significant cost reduction and a major improvement of the analytical performance.
With this next generation of serum free light chain assays, Sebia complements the existing range of electrophoresis tests for myeloma.
Supplier: Sebia
Website: