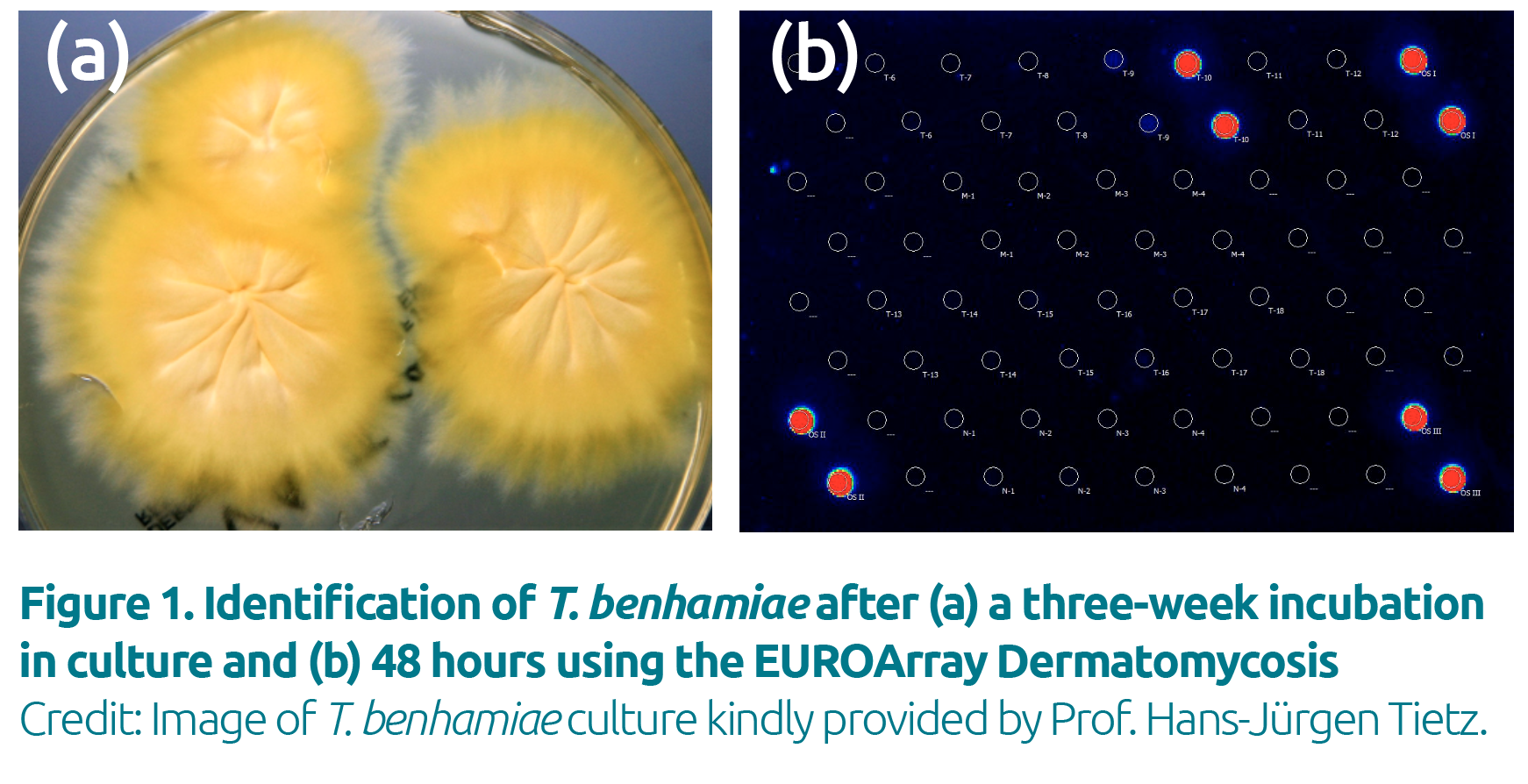
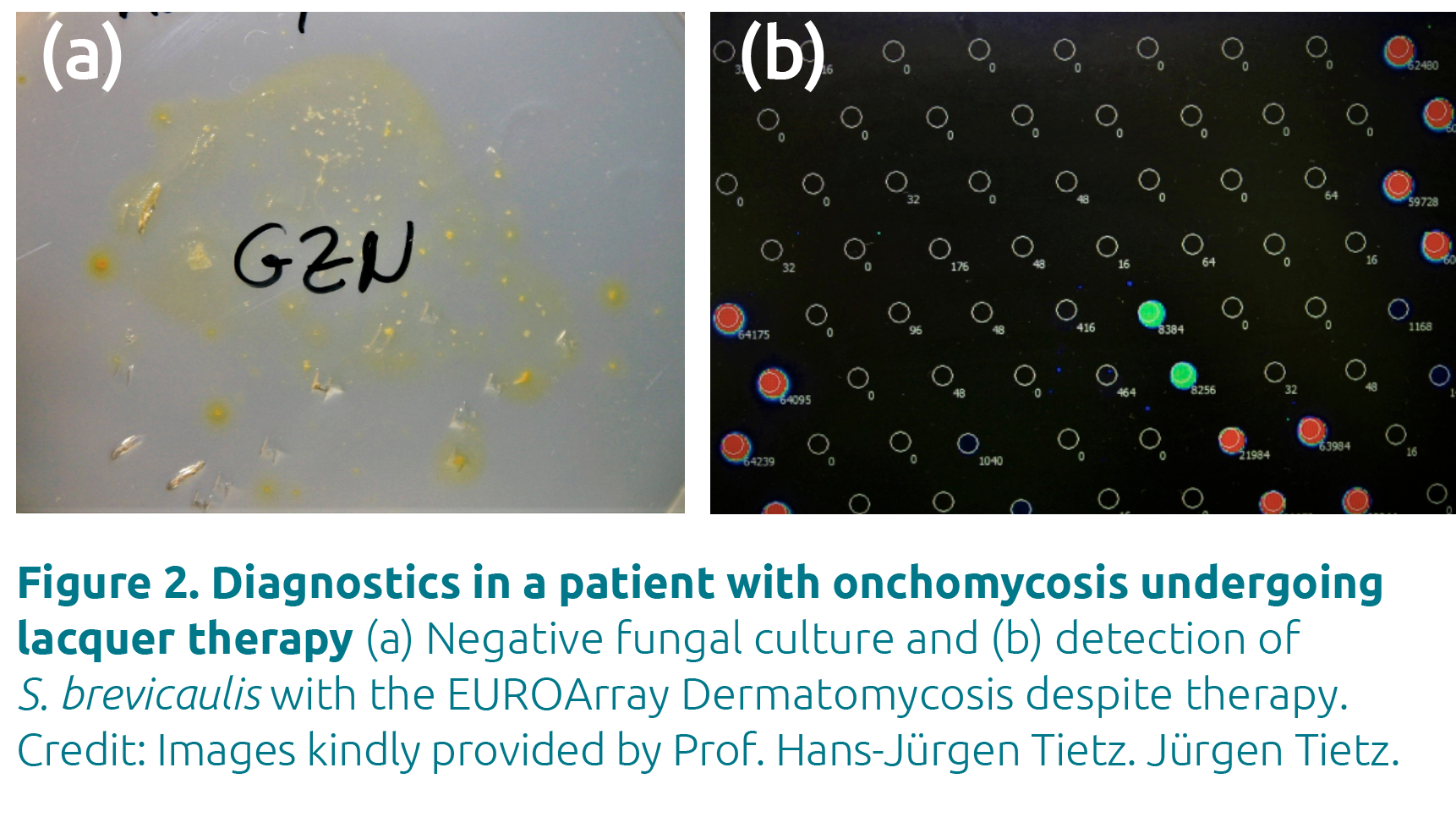
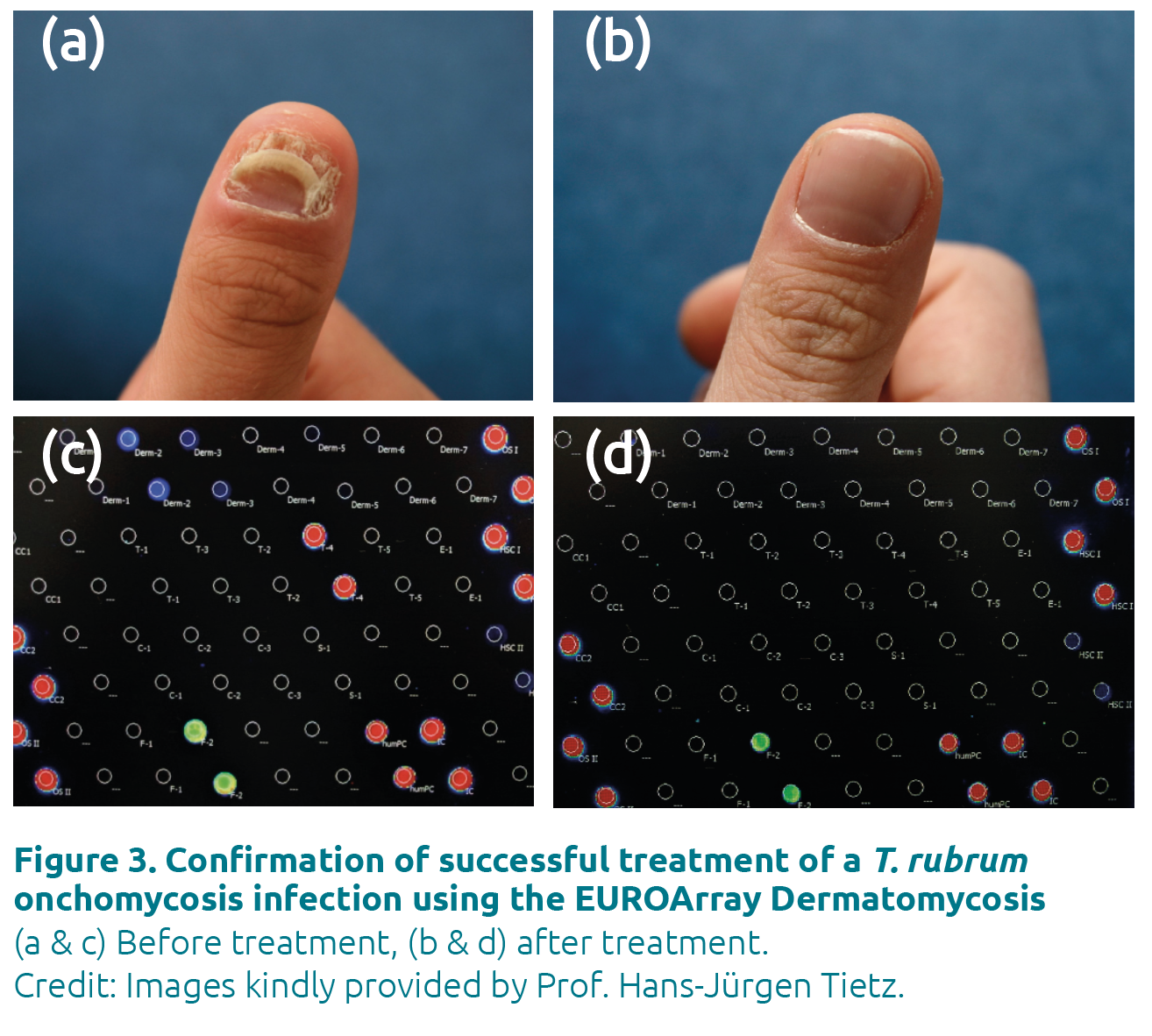
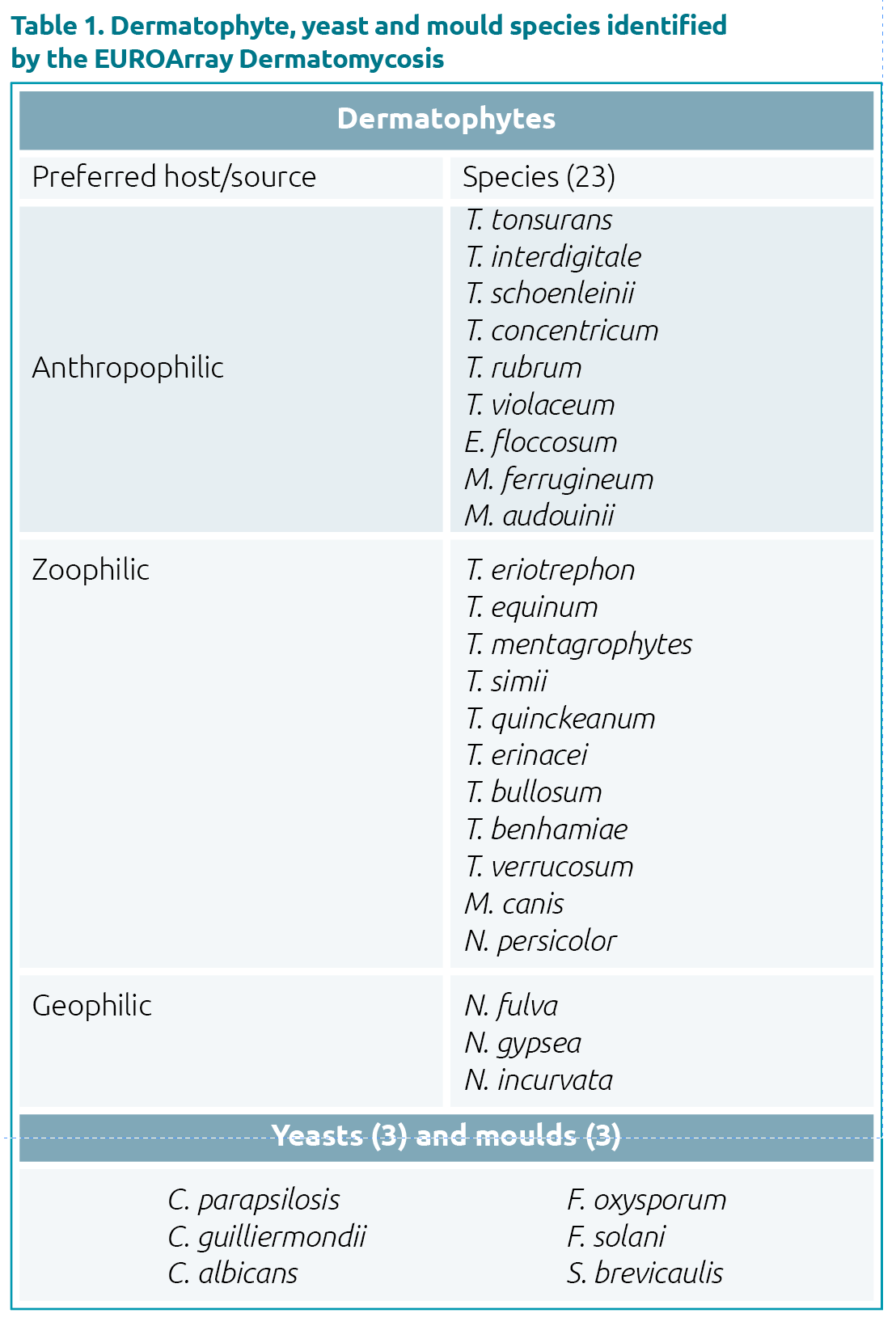
PCR allows rapid, differentiated dermatomycosis diagnostics
The diagnosis of fungal infections of the skin and nails has undergone a paradigm shift in recent years. Traditional culturing methods have in many cases been replaced by PCR-based diagnostics, which provide greater accuracy and yield results ina fraction of the time. The fast, differentiated detection of dermatomycosis pathogens enables prompt targeted treatment of infections.
Dermatomycosis
Dermatomycoses are fungal infections of the skin, hair and nails, which are caused predominantly by dermatophytes, but also by yeasts and moulds. They are among the most common infections worldwide, affecting an estimated 20–25% of the global population. Typical manifestations are white to yellowish nails or scaly, itching skin. Dermatophyte infections are referred to as tinea and classified according to the part of the body affected, for example feet (tinea pedis), nails (tinea unguium), scalp (tinea capitis) or trunk, neck, arms and legs (tinea corporis). Onychomycoses are nail infections caused by dermatophytes or yeasts/moulds. For effective treatment, dermatomycosis must be distinguished from other dermatological diseases such as psoriasis, keratoses, lichen planus, erythrasma, pityriasis rosea or seborrheic eczema. If left untreated, the infection may spread to different parts of the body or to close human contacts. Optimal therapeutic strategies also depend on precise identification of the pathogenic agent.
Dermatomycosis pathogens
Dermatophytes are divided into anthropophilic (infecting humans), zoophilic (infecting animals) and geophilic (residing in soil) species. Anthropophilic species are the most frequent causative agents of dermatomycosis, accounting for around 70% of cases. They are typically acquired via contact of bare skin with the pathogen, for example in swimming facilities or gyms. Zoophilic dermatophytes are usually transmitted to humans through intensive contact with pets, cows or horses, whereas geophilic dermatophytes can be transferred during gardening or farming activities. Yeasts and moulds are often part of the normal skin flora, but can also cause secondary infections at a dermatophyte infection site, where they spread and hinder treatment. The most clinically relevant dermatophytes belong to the genera Trichophyton, Epidermophyton, Nannizzia, and Microsporum, whereas human pathogenic yeast and moulds encompass Candida spp., Scopulariopsis brevicaulis and Fusarium sp. The most commonly detected dermatophyte pathogen is T. rubrum, followed by T. interdigitale.
New epidemiological developments
The spectrum of dermatomycosis pathogens is continually changing and expanding due to global tourism, migration and animal trading. An important emerging strain is T. benhamiae, which is now one of the most widespread zoophilic infections in Germany, having originally arrived via skinny pigs from Japan. The T. mentagrophytes subspecies type VII and type VIII (now known as T. indotineae), also migrated to Europe from Asia. Type VII is mainly sexually transmitted and is the first dermatophyte to be classified as a sexually transmitted disease. T. tonsurans was considered extinct in Germany until the 1990s, when it was reintroduced. It plagues practitioners of martial arts, spreading via gym mats, but is also increasingly transmitted via hairdressers and barbers. T. violaceum, T. soudanense and M. audouinii are also on the increase owing to migration of people.
Some mycoses have surged during the COVID-19 pandemic. An example is the increase in mycoses caused by anthropophilic pathogens such as T. rubrum, C. albicans and C. auris in COVID-19 patients. Zoophilic dermatomycoses have also flourished, especially in children, due to the widespread acquisition of pets during lockdowns. An example is tinea capitis caused by T. mentagrophytes or T. benhamiae caught from guinea pigs or rabbits.
Therapy and infection containment
Dermatomycosis therapy is based on a combination of topical ointments and systemic medication. Although broad spectrum antimycotics are generally used to treat fungal skin and nail infections, their effectiveness varies depending on the fungal species. Moreover, there is an increasing number of strains that are resistant to antimycotics, especially the normally very effective terbinafine. Therefore, accurate identification of the causative fungal species is important to enable selection of the most suitable therapy, especially given the often very lengthy treatment times. Infections that remain undetected or are insufficiently treated may persist for months and require systemic treatment. Tinea capitis in children must always be treated systemically to avoid scarring on the head.
Identification of the etiological agent is also important for infection containment. The detection of highly contagious anthropophilic species such as T. tonsurans,T. soudanense, T. violaceum or M. audouinii enables implementation of measures to prevent transmission to other people, for example in childcare or sports facilities. If a pet is pinpointed as the source of infection (e.g. T. mentagrophytes, T. benhamiae or T. quinckeanum), the animal can be treated in parallel to prevent further new or re-infections. Identification of infections picked up in the workplace, for example, in cattle breeders (T. verrucosum) or in gardeners (N. gypsea), may support employee compensation where relevant.
Limitations of conventional diagnostics
Classical detection methods for fungal infections are microscopic examination of infected materials (skin, nail, etc.) and fungal culture. Microscopy can identify the presence of a fungal infection, but not the pathogen causing it. It is also very time-consuming. Culturing allows identification of the infecting pathogen, but takes four to six weeks to yield a result and is often unsuccessful. As species identification in culture is based on morphology, it can also be difficult to achieve a clear result, especially for closely related fungal pathogens. Furthermore, antimycotic treatment that has already been initiated can hinder the growth of fungal cultures, leading to false negative results. For this reason, culturing is also unsuitable for therapy monitoring.
Advantages of PCR
PCR-based tests directly detect the genetic material of the pathogens and offer increased diagnostic sensitivity and specificity compared to classical procedures. PCR-based detection is particularly beneficial for rapid identification of dermatophytes that grow only extremely slowly in culture
(e.g. T. violaceum or T. verrucosum) or are difficult to identify morphologically (e.g. T. mentagrophytes or T. benhamiae; Fig. 1). PCR also enables reliable identification of mixed infections.
As results from PCR are not influenced by ongoing treatment, the method is suitable for diagnostics in patients who have already started therapy (Fig. 2).
Molecular diagnostic methods are also increasingly employed to assess the success of therapy, which cannot be achieved objectively with classical procedures. A negative PCR result indicates that the infection has been overcome (Fig. 3). Moreover, PCR is able to detect DNA from fungal spores, for example in onychomycosis, which can remain in tissue even after the infection has seemingly subsided. Such spores contain viable DNA and can germinate into virulent hyphae under favourable conditions, causing an endogenous relapse. With PCR, the complete elimination of the pathogen can be confirmed, thus avoiding premature termination of therapy.
Broad pathogen detection
The PCR-based detection should cover as wide a range of species as possible to avoid diagnostic gaps, especially given the rapidly expanding spectrum of dermatomycosis pathogens. The EUROArray Dermatomycosis (a microarray test) detects 50 dermatophyte species, and identifies 23 of the most relevant ones at species level, together with 3 yeasts and 3 moulds. The detected species are summarized in Table 1. In particular, the EUROArray is currently the only molecular diagnostic test that can differentiate between the genetically and morp-hologically similar species T. interdigitale and T. mentagrophytes.
Simple procedure
The PCR-based detection procedure is simple and can be established in any laboratory or medical practice. DNA is first isolated from skin scales, nail shavings or hair stubs using manual or automated procedures. Defined gene sections of the pathogens are then amplified by multiplex PCR and at the same time fluorescently labelled. The PCR products are hybridized to complementary probes on biochip microarray slides and detected using a proprietary scanner. The evaluation and interpretation of the results are fully automated with proprietary software and are thus objective. Various integrated controls ensure high result reliability. A detailed result report is produced for each patient and all findings are documented and archived digitally.
Conclusions
The progression of dermatophyte diagnostics from phenotype-based to genotype-based has paved the way for faster and more accurate identification of infection-causing pathogens. Rapid and comprehensive diagnostics enable timely implementation of appropriate treatment, increasing the chances of eliminating the fungal infection. Gene-based diagnostics also enable efficient tracking of pathogen spread, helping to stem outbreaks, especially of highly contagious fungi. Moreover, molecular diagnostics are increasingly used by veterinarians, enabling parallel treatment of infected pets to prevent further zoophilic infections. The incidence of dermatomycoses is increasing worldwide and will most likely continue to do so, especially given the growing populations of vulnerable individuals, such as the elderly and immunocompromised. PCR-based mycological diagnostics will play a central future role in identifying dermatophyte infections, guiding treatment and combatting epidemics.
The authors
Jacqueline Gosink PhD
EUROIMMUN, 23560 Lübeck, Germany
E-mail: j.gosink@euroimmun.de
For further information visit: www.derma-pcr.com
References
- Tietz HJ. Gentechnik revolutioniert Diagnostik [Genetic engineering revolutionizes diagnostic investigations]. Der Deutsche Dermatologe 2020;68(9) (in German).
- Trave I, Cozzani E, Canepa P et al. Real-life applicability of the EUROArray Dermatomycosis kit in the diagnosis of onychomycosis. Mycoses 2021;65(3):317–322 (https://doi.org/10.1111/myc.13405).
- Ständer S, Ha L, Kridin K et al. DNA-chip-based molecular testing as a clue for the diagnosis of tinea: a case series. J Eur Acad Dermatol Venereol 2021;35(8):e522–e524.
- Further case studies are described in a series of articles by Prof. Hans-Jürgen Tietz (Director of the Institute of Fungal Diseases and Internal Medicine, Berlin, Germany). EUROIMMUN
(https://www.dermatophyte-pcr.com/physicians-laboratories/article-series-by-prof-tietz.html).