Performance studies indicate suitability of VERIS* for routine lab applications
Beckman Coulter’s VERIS Molecular Diagnostic (MDx) System* is a fully automated system for quantitative and qualitative analysis of molecular targets. It integrates sample introduction, nucleic acid extraction, reaction setup, real-time PCR amplification and detection, and results interpretation. Abstracts of studies presented as posters at the recent European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Barcelona highlighted the suitability of the system and its assays for use in the routine laboratory. Three of these abstracts appear below.
VERIS Molecular Diagnostic System sample-to-sample crossover contamination study
Summary
Sample carry-over and cross-contamination present a high risk for the laboratory. VERIS was developed to have a false positive rate due to cross contamination of less than 1 in 500 tests with an overall design goal of zero. This study involved several stages: to assess the sample-to-sample contamination rate using a real-time PCR assay and then characterize potential sources of contamination. System modifications were then to be developed to resolve any carry-over and cross-contamination and the system then retested.
Method and Results
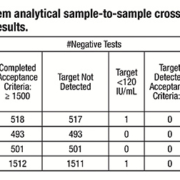
Contamination characterization was performed by swabbing areas of the instrument before and after running a series of high concentration level positive samples to determine potential sources of contamination. Twenty high positive Cytomegalovirus (CMV) spiked samples at a concentration of 1×1010 IU/mL were used in this testing. A total of 27 high risk areas on the instrument were evaluated. High risk areas were defined as areas on the system where liquid handling occurs and where potential splashing of sample or reagents can occur. The swabs were placed in the TE buffer and then processed on the VERIS system. Swab assessments identified several areas where splashing and contamination of high positive samples was occurring, with the potential to contaminate a future sample.**
This resulted in several modifications to the liquid handling parameters and motor speeds to eliminate the potential for sample-to-sample contamination. Swab testing was used to verify the effectiveness of the modifications. The study concluded that accurate results for true negative samples were now being shown, with no detectable carryover and contamination from high positive to negative samples. This was true when the concentration of CMV target in the samples was above clinical levels and the frequency of high positives in the sample population exceeded 30%.
Cytomegalovirus (CMV) viral load assay for the VERIS MDx System
Summary
The initial assay menu includes the VERIS Cytomegalovirus (CMV) Assay*, intended for use in conjunction with clinical presentation and other laboratory markers as an aid in monitoring CMV viral load and for the detection of virus reactivation. This study reported on performance in key analytical and clinical measures.
Method and Results
137 paired samples tested on both the VERIS CMV Assay and the Roche COBAS AmpliPrep/COBAS TaqMan CMV Test were used to demonstrate method comparison in accordance with CLSI EP9-A2. 287 specimens were tested to demonstrate the clinical specificity of the assay.
Assay measuring interval: the assay is linear for human CMV with a lower limit of quantitation (LLQ) of 120 IU/mL and an upper limit of quantitation (ULQ) of 10,000,000 IU/mL. A nine member panel of the CMV AD169 reference strain demonstrated a linear range of 159 to 13,400,000 IU/mL (2.20-7.13 Log IU/mL). A four-member panel of the 1st WHO International Standard for Human Cytomegalovirus (HCMV) (NIBSC 09/162) demonstrated a linear range from 120 and 10,000 IU/mL (2.08-4.00 Log IU/mL)
Precision: demonstrated a total (within-run and between-run) standard deviation of less than 0.15 Log IU/mL across its linear range.
Sensitivity: The VERIS CMV Assay was shown to have a Limit of Detection (LoD) of 30 IU/mL (1.48 Log IU/mL) across all subtypes tested.
Performance evaluation of the Beckman Coulter VERIS Cytomegalovirus Assay on the VERIS MDx System
Summary
Another study assessed the VERIS CMV assay for reproducibility and specificity, comparing it with the Roche COBAS AmpliPrep/COBAS TaqMan CMV test. In immune-compromised individuals, the activation of the latent virus, is a significant cause of morbidity and mortality. Real-time polymerase chain reaction (PCR) assays offer the ability to diagnose active infection and monitor those individuals at risk.
Method and Results
Using paired plasma samples, total assay imprecision was
≤ 4.6% CV with the SD ≤0.14. Clinical specificity with negative samples was 100% with a lower bound of the 95% CI = 98.7%. It had comparable recoveries to the Roche assay with a Passing-Bablock regression equation of VERIS=0.30+1.00 (Roche), r=0.88 and n=130.
*Not for sale or distribution in the U.S.; not available in all markets.
**References available on request