Pharmacogenetic testing of CYP2C19 associated with clopidogrel therapy
Because of the critical nature of the reported clinical events (such as stent thrombosis and hemorrhage) associated with patients with certain cytochrome p450 2C19 (CYP2C19) variants receiving clopidogrel therapy, additional clinical studies are warranted. Large scale clinical trials seeking to correlate patient’s response to clopidogrel (Plavix®) with the CYP2C19 genotype may benefit from the inclusion of the CYP2C19 genotyping results into therapeutic anti-platelet therapy decisions before percutaneous coronary intervention.
by Dr H. Han, K. Blakely and Dr S. Lewis
Clinical potential of pharmacogenetic testing
The clinical potential of pharmacogenetic testing has increasingly been shown to influence treatment effectiveness for a number of therapies including tyrosine kinase inhibitors (TKIs) such as imatinib directed at chronic myelogenous leukemia (CML), gefitinib and erlotinib targeting the epidermal growth factor receptor (EGFR) in lung and other cancers and clopidogrel, an anti-platelet therapy prescribed for patients receiving percutaneous coronary intervention (PCI) [1–4].
Using genetic information in a clinically beneficial fashion, that is providing evidence-based data to show that the genetic information provided to the health care team is clinically applicable to diagnosis, treatment and prognosis and fiscally responsible, is challenging. Despite numerous studies relating genetic variants to clinical effects, the integration of genetics into routine clinical practice is restricted [5]. Success has been demonstrated especially in the area of oncology and many patients receive cancer therapy guided by genetic testing. Some examples of cancers that have therapy that may be guided by molecular testing include breast, lung, colon and leukemia. Obstacles to integration of genetic testing into routine practice include reimbursement issues and accessibility. Of particular concern to the cardiology team is the past unavailability of rapid point-of-care genotype testing for cytochrome p450 2C19 (CYP2C19) variants during PCI. Molecular genetic testing is typically performed at reference laboratories and the results of the testing have not been available to cardiologists at the time of PCI.
Moving to a state of personalized medicine and genome-guided care requires a number of important steps:
- Initially, single nucleotide polymorphisms (SNPs) must be discovered in the normal population. For the CYP2C19 gene, this includes greater than 2075 of the referenced SNPs.
- Secondly, SNPs must be related to phenotyped subjects in functional studies. For CYP2C19, currently the three clinically relevant variants appear to be *2,*3 and *17.
- Findings must be replicated in clinical settings demonstrating the advantage of providing the CYP2C19 genotype to guide practice at actionable times.
- Additional environmental, clinical and other genetic considerations must be evaluated to move to the personalized medicine of CYP2C19 drugs. In the case of CYP2C19 metabolized medications, a number of drugs may be prescribed to patients receiving PCI, including clopidogrel, omeprazole and aspirin. Both clopidogrel and omeprazole have narrow therapeutic ratios [6–9].
Clopidogrel therapy and genetic testing
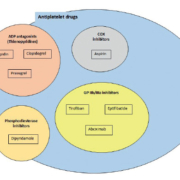
The CYP2C19 enzyme is responsible for the metabolism of approximately 15% of all prescription drugs including anti-platelet therapies such as clopidogrel, beta-blockers (propranolol), anti-depressants (imipramine), anti-convulsants (phenytoin) and proton pump inhibitors (omeperizol) [6]. Of particular interest to cardiologists is anti-platelet therapy. Anti-platelet therapy may be classified according to the target of action and includes ADP antagonists (clopidogrel, prasugrel and ticlopidin), COX inhibitors (aspirin), phosphodieterase inhibitors (dipyridamole) and GP IIb/IIIa inhibitors (tirofiban, eptifibatide, abcixmab) shown in Figure 1. Clopidogrel bisulfate is a thienopyridine irreversible inhibitor of ADP-induced platelet aggregation by directly preventing ADP binding to its receptor P2Y12 and thereby preventing subsequent activation of the of the glycoprotein IIb/IIIa complex (Fig. 2). Platelets are irreversibly inactive for the remainder of their life, approximately 7–10 days [6].
Metabolism of clopidogrel (Fig. 3) occurs in the liver by several p450 enzymes. CYP2C19 is involved in the formation of the primary inactive metabolite, 2-oxo-clopidogrel and the active thiol clopidogrel deritive. Anti-platelet effects may be seen 2 hours after oral administration with a steady state inhibition reached between days 3–7. Anti-platelet effects are dose dependent and may be measured by platelet aggregation assays and differ according to CYP2C19 genotype. This association of CYP2C19 genotype and clopidogrel treatment outcome was evaluated in several clinical trials [2, 10].
Variants of the CYP2C19 enzyme include both reduced drug metabolism variants (*2,*3,*4,*5) and increased drug metabolism variants (*17). Most patients undergoing the insertion of a drug eluting stent after myocardial infarction are prescribed clopidogrel bisulfate (Plavix ®) and aspirin as anti-platelet therapy. Numerous investigations of patients prescribed the anti-platelet drug clopidogrel bisulfate (Plavix®) have demonstrated relationships between the patient’s CYP2C19 genotype and their response to clopidogrel as related to clinical outcomes such as blood clots, stent thrombosis, bleeding, myocardial infarctions and major cardiovascular events (MACE). Loss of function CYP2C19*2 and *3 variants have been associated with higher levels of ADP-induced platelet aggregation in patients receiving clopidogrel therapy, and therefore have a greater risk of major cardiovascular events, including stent thrombosis. Several clinical variables are implicated in the platelet response to clopidogrel, but the strongest predictor is the loss of function CYP2C19*2 allele. Approximately 30% of western European individuals, and 50% of Asian individuals carry the *2 allele and studies have associated the *2 allele with a significant increased risk of adverse cardiovascular events and stent thrombosis [11, 12].
In March of 2010, the FDA announced a boxed warning to the clopidogrel label, alerting patients and clinicians that clopidogrel may be less effective in patients carrying the reduced function alleles [13].
The CYP2C19*17 gain-of-function allelic variant was shown to significantly reduce ADP-induced platelet aggregation in clopidogrel-treated patients and was therefore predicted to confer an increased risk of bleeding. CYP2C19*17 alleles are found in the United States population ranging from <5% homozygotes to ~40% heterozygotes. The most common CYP2C10*17 variant, -806C>T, is associated with recruitment of a transcription factor to the mutated site, enhancing transcription and expression of the CYP2C19 enzyme. [14]
Current and future options for CYP2C19 testing
Currently, molecular reference laboratories can provide CYP2C19 genotyping results in several days. Recently, the Spartan RX point-of-care instrument was FDA cleared for in vitro diagnostic testing. This platform uses buccal cells and can provide results in about one hour [15].
Because of the past inability of molecular laboratories to provide cardiologists with rapid point-of-care testing for CYP2C19 variants, large-scale clinical trials with rapid genetic results provided to clinicians at the time of the PCI have been limited. Currently, a clinical trial (NCT01742117) is sponsored by the Center for Individualized Medicine at Mayo Clinic and is entitled ‘Tailored Antiplatelet Initiation to Lessen Outcomes due to Clopidogrel Resistance after Percutaneous Coronary Intervention’ (TAILOR-PCI). This randomized prospective study will use the FDA-cleared Spartan RX to genotype CYP2C19 for the *2,*3 and*17 alleles in cardiac stent patients. The carriers will receive ticagrelor instead of clopidogrel. Approximately 6000 patients will be enrolled in this large study with a completion date of June 2016. Results of this study should provide additional risk stratification and treatment decisions. Additional trials to formulate strategies for the most effective and cost efficient anti-platelet treatment will hopefully follow [15].
Advances in next-generation sequencing (NGS) are providing the promise of available genetic information on patients at rapidly reduced costs. Clinical applications of NGS will hopefully include pharmacogenetic information about patients that may be accessed by clinicians through the electronic medical record to provide immediate guidance of optimum therapy for not only anti-platelet therapy but for numerous other medications.
References
1. Gurbel PA, Tantry US. Controversies in cardiovascular medicine. Platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents. Circulation 2012; 125: 1276–1287.
2. Sibbing D, Koch W, Gebhard D, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 2010; 121: 512–518.
3. Krishna V, Diamond GA, Kaiil S. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents?: the role of platelet reactivity and genotype testing in the prevention of atherothrombotic cardiovascular events remains unproven. Circulation 2012; 125: 1288–1303.
4. Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Eng J Med. 2009; 360: 354–362.
5. Roberts JD, Wells GA, Le May ML, et al. Point-of-care genetic testing for personalization of antiplatelet treatment (RAPID GENE): a prospective, randomized, proof-of-concept trial. Lancet 2012; 379: 1705–1711.
6. Plavix package insert. Bristol-Myers Squibb 2009.
7. Tantry U, Kereiakes D, Gurbel P. Clopidogrel and proton pump inhibitors. influence of pharmacological interactions on clinical outcomes and mechanistic explanations. JACC Cardiovasc Interv. 2011; 4: 365–380.
8. Gurbel PA, Tantry US, Shuldiner AR. Letter by Gurbel et al. regarding article, “Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement”. Circulation 2010; 122: e478.
9. Sibbing D, Koch W, Gebhard D, et al. Response to letter regarding article, “Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement”. Circulation 2010; 122: e479.
10. Taubert D, Kastrati A, Harlfinger S, et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thomb Haemost. 2004; 92: 311–316.
11. Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009; 360:363–375.
12. Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310: 189–198.
13. Holmes DR Jr, Dehmer GJ, Kaul S, et al. ACCF/AHA Clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010; 56: 321–341.
14. Rudberg I, Mohebi B, Hermann M, et al. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther. 2008; 83: 322–327.
15. Spartan Bioscience announces 6,000-patient study of personalized medicine for cardiac stents. PRWeb 2012. (http://www.prweb.com/releases/2012/12/prweb10252073.htm)
The authors
Heping Han MD, PhD, MB (ASCP);
Katherine Blakely BS; Sally Lewis* PhD, MLS (ASCP), MB
Tarleton State University, Fort Worth,
TX 76104, USA
*Corresponding author
E-mail: slewis@tarleton.edu