Point-of-care diagnostics for malaria
In spite of increased publicity in the Western world about malaria and drives to provide mosquito nets, the disease is still endemic in a large part of the world. This article discusses different methods of malaria diagnosis and the role that point-of-care tests can play in the ultimate goal of malaria elimination.
by Dr Jackie Cook
Finding the balance: over- and under-diagnosing malaria
Malaria remains a huge burden in many parts of the world, particularly in sub-Saharan Africa. Despite increased availability of effective treatments and interventions, malaria elimination is still out of reach for many countries. Whilst availability of effective interventions that reduce contact with infected mosquitoes, such as insecticide treated bed-nets or indoor spraying with insecticide are key to reducing malaria prevalence, case management also plays a key role. Many who need treatment are unable to get it, either through lack of access to healthcare, or because infections remain undiagnosed. Conversely, some studies suggest that many patients are receiving anti-malarials unnecessarily due to a tendency to diagnose based solely on clinical symptoms, many of which are similar to other infections, rather than using a diagnostic. In under-resourced settings, this can result in any child presenting with a fever being prescribed malaria drugs. This simultaneously means non-malaria fevers remain undiagnosed and untreated, as well as a large proportion of unnecessary prescriptions for malaria drugs, which increases healthcare costs and the risk of drug resistance, a very potent threat. In order to counteract this, the last few decades have brought a push from health officials, researchers, donors and governments alike to confirm every suspected case of malaria before prescribing treatment.
Microscopy
For many, malaria diagnosis is performed using microscopy, a procedure that is relatively cheap but requires a skilful operator. Malaria is caused by the plasmodium parasite and it undergoes several developmental and replication stages in the human. These stages can be seen through a microscope when blood is prepared on either a thin or thick film and stained, normally, with Giemsa or Wright’s stains. Experienced microscopists can detect down to 1 parasite per microlitre of blood, although the typically quoted sensitivity for microscopy is approximately 100 parasites per microlitre. In reality, the sensitivity of the test depends greatly on the microscopist. In areas where malaria transmission is declining, microscopists can go months without seeing a positive slide, and as such, skills may begin to decline. In addition, the need for well-maintained microscopes and access to slides and stain can mean microscopy is not always available.
Rapid diagnostic tests
The first malaria rapid diagnostic test (RDT) was developed in 1993 and in the decades since many variations have proliferated on the market. RDTs are typically immunochromatograhic tests that use monoclonal antibodies to detect the presence of plasmodium antigens (proteins produced by the parasite) which are present in the blood of infected, or recently infected, individuals. They are generally stable at a range of temperatures and do not require special storage conditions. RDTs require significantly less training for use than microscopy and a positive infection is easy to identify by visualization of a ‘positive’ line, meaning the results are much less subjective. Most RDTs require 15–20 minutes for development, meaning treatment can be given while patients wait at health facilities.
However, there are a few downsides to the use of RDTs. The presence of parasite antigen doesn’t always equate with a current infection, but can signify a recently cleared infection from within the previous two weeks. In addition, several studies have reported the deletion of certain antigens detected by RDTs in plasmodium parasites, meaning false-negative results may be obtained in areas using these types of RDTs [1]. The World Health Organization (WHO), in collaboration with the Foundation for Innovative New Diagnostics (FIND), has set up an RDT product testing programme, an essential quality assurance component considering the huge influx of RDT brands that have popped up in the past 20 years [2]. The reports from the programme make worrying reading with very low sensitivity for some brands, differences between batches of RDT and a general lower sensitivity for non-falciparum infections for nearly all brands.
The hidden reservoir: asymptomatic, low-density infections
In general, the limited sensitivity of both microscopy and RDT (unreliable detection in infections with a parasite density less than 100 parasites per microlitre) is not an issue for symptomatic malaria infections, the majority of which will consist of high parasite densities. However, asymptomatic infections are numerous, in high and low transmission settings. These asymptomatic infections pose a problem for control programmes. The carriers do not feel unwell so have no reason to present to a health facility for testing and yet, they may be infectious to mosquitoes, meaning they pose a risk for onward transmission. In order to detect and treat these asymptomatic infections, malaria programmes are now taking their diagnostics into the community in a strategy termed Mass Screening and Treatment (MSAT). This involves testing everyone within a community regardless of whether they have symptoms. Many of these infections are asymptomatic and therefore also likely to be low-density; hence which test you use can mean the difference between detecting 10 infections or 100 infections. Whilst RDT is ideal for field conditions, studies have shown that they can miss a large proportion of infections that are present [3].
Molecular tests
Polymerase chain reaction
More sensitive diagnostics are available in the terms of molecular tests. The most commonly used is polymerase chain reaction (PCR). Numerous PCR assays have been developed, many based on amplifying the 18S ribosomal RNA (18SrRNA), first published by Snounou and colleagues in 1993 [4]. PCR detects parasite nucleic acids and can detect much lower parasite densities than RDT or microscopy, with tests reportedly able to detect down to 1 parasite per microlitre of blood, as well as being able to accurately distinguish between plasmodium species. However, the number of assays available has resulted in calls for a standardized test so results can be compared across the world. PCR tests are generally performed on blood collected on filter paper but the equipment required for PCR and the expense of maintaining a sterile lab environment precludes PCR from being available in many health facilities. This means that samples need to be sent away, with an often long wait for results. Although more field-friendly PCR methods are in the pipeline, currently, PCR is not generally considered suitable for a point-of-care test, although it’s use in epidemiological studies is undisputed.
Loop-mediated isothermal amplification
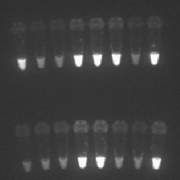
Loop-mediated isothermal amplification (LAMP) was first developed in 2000, with the aim to amplify DNA in a sensitive, specific and speedy manner (Figs 1, 2). One of the main advantages is the fact it can be performed under isothermal conditions, and thus averting the need for a thermocycler. LAMP can be thought of as a ‘rough-and-ready’ PCR, as it is also less sensitive to inhibitors present in biological samples, and therefore allows the use of simple and cheap DNA extraction methods. The fast time-to-results and the minimal equipment required make LAMP an attractive option for field diagnosis. In order to make this a viable option, FIND and partners Eiken Chemical Ltd, Japan, and the Hospital for Tropical Diseases (HTD), London, UK have developed a field-stable kit with all reagents freeze-dried into the lid of the reaction tube, which means minimal processing is required. Although still in the development and testing stage, current results of the use of the kit are promising, with strong agreement with PCR results and a considerably higher sensitivity than RDT [5-8]. Whilst seemingly the most sensitive of the point-of-care tests available, there are some downsides to LAMP. Results still take considerably longer than RDT, requiring patients to wait at clinics for 2 hours for results, or leaving the health facility staff with the complicated task of contacting and following up any positive patients. In addition, electricity is required for the processing of samples, making it not practical for many places.
Future for point-of-care diagnostics for malaria
These advances in molecular diagnostics mean infections that would previously have remained undetected can now be confirmed, treated and cleared. Identifying and treating all infections becomes a greater priority as transmission reduces and the possibility of elimination comes into focus. This is occurring in areas around the world such as Swaziland and Zanzibar in Africa and in South East Asia, where the need to eliminate has become ever more important with the emergence of drug-resistant parasites. In these areas, identification of every last parasite is the aim and development of a quick, sensitive and reliable diagnostic is key to that.
As more studies reveal the extent of the low-density parasite reservoir, there is a sense of ‘the more we look the more malaria we will find’. But do we need to find all these infections in order to eliminate malaria? It should be noted that these ‘super-sensitive’ tests are a relatively recent phenomena and that countries have succeeded in malaria elimination without them. The role these low-density parasitemias play in transmission is not fully understood but for now the aim remains to clear the last parasite standing.
References
1. Houze S, Hubert V, Le Pessec G, Le Bras J, Clain J. Combined deletions of pfhrp2 and pfhrp3 genes result in Plasmodium falciparum malaria false-negative rapid diagnostic test. J Clin Microbiol. 2011; 49(7): 2694–2696.
2. WHO, FIND, CDC. Malaria rapid diagnostic test performance: Results of WHO product testing of malaria RDTs: Round 5. 2013; http://www.who.int/malaria/publications/atoz/9789241507554/en/.
3. Cook J, Xu W, Msellem M, Vonk M, Bergström B, Gosling R, Al-Mafazy AW, McElroy P, Molteni F, Abass AK, Garimo I, Ramsan M, Ali A, Mårtensson A, Björkman A. Mass screening and treatment on the basis of results of a plasmodium falciparum-specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis. 2015; 211(9): 1476–1483.
4. Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993; 61(2): 315–320.
5. Hopkins H, González IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, Agaba B, Kyabayinze DJ, Sutherland CJ, Perkins MD, Bell D. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis. 2013; 208(4): 645–652.
6. Polley SD, González IJ, Mohamed D, Daly R, Bowers K, Watson J, Mewse E, Armstrong M, Gray C, Perkins MD, Bell D, Kanda H, Tomita N, Kubota Y, Mori Y, Chiodini PL, Sutherland CJ. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis. 2013; 208(4): 637–644.
7. Aydin-Schmidt B, Xu W, González IJ, Polley SD, Bell D, Shakely D, Msellem MI, Björkman A, Mårtensson A. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS One 2014; 9(8): e103905.
8. Cook J, Aydin-Schmidt B, González IJ, Bell D, Edlund E, Nassor MH, Msellem M, Ali A, Abass AK, Mårtensson A, Björkman A. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J. 2015; 14(1): 43.
The author
Jackie Cook PhD
London School of Hygiene and Tropical Medicine, London, UK
E-mail: Jackie.cook@lshtm.ac.uk