Point-of-care testing for HbA1c: clinical need and analytical quality
HbA1c plays an essential role in the diagnosis and management of people with diabetes. Point-of-care testing for HbA1c offers a wealth of opportunities to provide a rapid, accurate and easy to access tool for healthcare professionals, with performance of some devices matching or even outperforming routine laboratory instruments.
by Dr Emma English, Larissa-Nele Schaffert and Dr Erna Lenters-Westra
Introduction
Diabetes mellitus (DM) represents a major health problem of the 21st century, causing severe long-term damage to the cardiovascular and nervous system as well as the eyes and kidneys. The International Diabetes Federation (IDF) estimates that currently 425 million people globally, have diabetes. Regions such as Africa are predicted to see an increase in diabetes cases of over 150 % by the year 2045, representing a huge burden on already limited health resources [1].
Hemoglobin A1c (HbA1c) has traditionally been used to monitor glycemic control in patients with diabetes. Multiple large-scale studies have demonstrated the benefit of lowering HbA1c values in reducing microvascular and macrovascular complications. HbA1c is formed by glycation of the N-terminal valine of the beta chain of hemoglobin, which is a non-enzymatic reaction occurring within red blood cells, resulting in an increased negative charge of the molecule. The more glucose that is present in the blood stream during the lifetime of the red blood cells (around 100–120 days), the higher the concentration of HbA1c.
In 2011 the World Health Organization (WHO) advocated the use of HbA1c for the diagnosis of type 2 DM (T2DM) and this has been implemented in a number of countries worldwide. The threshold for diagnosing T2DM was determined as 48 mmol/mol (6.5 %) HbA1c, although this value has not been universally accepted [2].
The typical clinical procedure to assess patients with suspected diabetes will often involve a risk score to assess risk factors for diabetes such as age, family history and BMI and if this is elevated an HbA1c test may be requested. The testing process involves at least two appointments with a GP/practice nurse: (1) blood samples being taken during the first visit, and (2) 1–2 weeks later results being discussed with the patient, after laboratory analysis. If elevated HbA1c levels are found and there are no other symptoms then a repeat HbA1c test would normally be undertaken, adding to the length of time taken to reach a diagnosis.
Why are HbA1c point-of-care tests useful?
There are a number of potential benefits to using point-of-care testing (POCT) for HbA1c. The timely identification of disease is a key advantage of POCT as it provides immediate results at the time of patient consultation; this enables decisions to be made at the earliest possible opportunity, potentially resulting in fewer patient visits. It should be noted, however, that there are currently no guidelines supporting the use of POCT devices for the diagnosis of diabetes. In addition to potential use for diagnosis, the regular monitoring of people with diabetes may be more effectively facilitated with POCT devices, especially in rural or hard-to-reach environments. The patient may have their HbA1c levels tested upon arrival at clinic and the results will be available at the consultation, saving the need for a pre-visit. Alternatively the analysis may be undertaken during the consultation itself and the analysis time can be used to perform other measurements, such as blood pressure, or provide an opportunity for the clinician to engage in patient education.
The Noklus programme is an excellent example of where POCT has been shown to be effective. Owing to its geography, Norway has a low population density, resulting in many patients having to travel long distances to access primary healthcare provision. Repeated visits to the healthcare providers are time consuming and costly and ideally avoided. The use of POCT could mitigate some of the need to travel; indeed Norway has been using HbA1c POCT for more than 17 years for monitoring patients with diabetes and for the last two years, it has been used for the diagnosis of T2DM [3]. Recently Noklus have expanded activities to include the use of pharmacies to identify those at risk of diabetes and to test for diabetes using a POCT device, demonstrating a clearly expanding role for POCT [4].
The area where the diabetes disease burden is increasing at the fastest rate is in sub-Saharan Africa. Current estimates predict a threefold increase in cases over the next 25 years with four out of five diabetes-related deaths occurring in those of working age below 60 years [1]. This is a high priority region for early identification of disease and early intervention to limit progression of complications, as the costs associated with diabetes care are beyond the reach of many countries in this region. With two-thirds of those with diabetes unaware that they have the disease, access to rapid, easy-to-use and portable HbA1c devices is needed. POCT devices are likely to play a crucial role in the identification and monitoring of people with diabetes in Africa, especially as the current laboratory infrastructure is unlikely to meet this need [5].
HbA1c measurement
Analytical methods are based on either differences in structure, or charge of the glycated versus non-glycated hemoglobin. The main methods used for POCT are:
- Cation exchange chromatography
Hemoglobin species (HbA1c and HbA0) are separated according to the difference in isoelectric point, by employing differences in ionic interactions between the hemoglobin in the blood sample and the cation exchange groups on the column resin surface. - Immunoassay
The immunoassay method uses antibodies that bind to the N-terminal glycated tetrapeptide or hexapeptide group of the HbA1c, forming immunocomplexes that can be detected and measured using a turbidimeter or a nephelometer. - Affinity chromatography
Affinity chromatography is a separation technique based on structural differences between glycated versus non-glycated hemoglobin, which utilizes m-aminophenylboronic acid and its specific interactions with the glucose adduct of glycated hemoglobin. - Enzymatic assay
Enzymatic quantification of HbA1c is based on cleavage of the beta chain of hemoglobin by specific proteases to liberate peptides, which then further react to produce a measurable signal.
Most POCT devices for HbA1c use a drop of capillary whole blood, collected via the finger-prick procedure. Following application to the test cartridge, the sample is analysed within a few minutes, although some methods require additional preparation steps. Details of current devices are available from manufacturers and in Schaffert et al. [6].
Quality criteria for HbA1c POCT
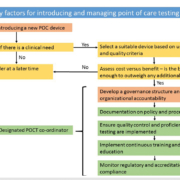
WHO guidance states that HbA1c may be used for diagnosis of T2DM provided “stringent quality assurance tests are in place and assays are standardized to criteria aligned to the international reference values”[2]. For laboratory-based methods, the quality standards for HbA1c as a diagnostic tool and HbA1c as a monitoring tool are the same. Quality targets vary, depending on the organization or body giving the guidance; however, the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) recently proposed the use of sigma metrics to define and set quality targets that can be adjusted depending on the specific requirements of the system/setting being assessed [7]. Currently there is no further additional guidance that specifically relates to quality targets for POC devices for HbA1c. What is essential for high quality in POCT is a robust quality framework, see Figure 1.
Assessing the quality of POC devices
There are several ways in which the quality of an analytical device can be assessed. A common approach is a laboratory evaluation following standardized protocols, such as the Clinical & Laboratory Standards Institute (CLSI) guidelines. To meet WHO criteria, such evaluations should be undertaken using samples targeted to the Reference Measurement Procedure (RMP), which for HbA1c is the IFCC RMP. The results of the evaluation will provide a set of performance figures for that instrument. In order to interpret these values, quality targets or criteria also need to be applied. In 2015, HbA1c was one of the first analytes for which such quality criteria have been set and these criteria are based on sigma metrics [7]. A significant number of method evaluations for HbA1c POC devices have been undertaken in recent years and the findings of these have been summarized in a recent systematic review and meta-analysis [8]. More recently sigma metrics have been applied alongside CLSI guidance [9].
Another approach is to evaluate external quality assessment (EQA) data, which provides a ‘real world’ perspective on method performance. A recent large-scale study by the IFCC demonstrated that performance of HbA1c testing varies between countries and between manufacturers but also showed that performance can vary between countries with a single manufacturer and method type [10].
HbA1c POCT myths and facts
There is often controversy around hot topics such as the use of HbA1c testing for the diagnosis of T2DM and in particular the use of POC for diagnosis; however, there are some key messages to consider:
- Myth: POCT devices do not perform well in the hands of non-laboratory users. In fact, the evidence available indicates that performance of devices is no different between laboratory and non-laboratory personnel [8].
- Myth: POCT quality targets are different to laboratory instruments. There are no criteria specifically for POCT devices and the international quality targets are aimed at both laboratory devices and POCT devices [7].
- Myth: POCT never performs as well as laboratory analysers. Although there are studies that show that POCT devices do not meet quality criteria [11], in general they perform no better or no worse than laboratory analysers [12].
- Fact: POCT devices play an important role in healthcare provision in hard-to-reach environments [4].
- Fact: POCT devices are increasingly used in national screening programmes owing to their ease of use and less invasive nature (finger prick versus venipuncture) [13].
- Fact: Industry, scientific organizations, healthcare policy makers and non-governmental organizations need to work together to provide, low cost, robust and accurate HbA1c POC testing in order to tackle the rapidly increasing global burden of diabetes.
Summary
HbA1c POC devices play a valuable role in tackling the global diabetes epidemic, offering rapid and accurate test results, which have the potential to improve patient care and timeliness of diagnosis and treatment changes during monitoring of glycemic control. Quality guidelines are the same for POCT devices as laboratory devices and many POCT devices perform as well as laboratory instruments. Essential to all high quality testing is a robust EQA scheme and adequate training for all users.
References
1. IDF Diabetes Atlas, 8th edn. International Diabetes Federation (IDF) 2017 (http://www.diabetesatlas.org).
2. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. World Health Organization 2011 (http://www.who.int/diabetes/publications/report-hba1c_2011.pdf).
3. Skeie S, Thue G, Sandberg S. Use and interpretation of HbA1c testing in general practice. Implications for quality of care. Scand J Clin Lab Invest 2000; 60(5): 349–356.
4. Risøy AJ, Kjome RLS, Sandberg S, Sølvik UØ. Risk assessment and HbA1c measurement in Norwegian community pharmacies to identify people with undiagnosed type 2 diabetes – A feasibility study. PLoS One 2018; 13(2): e0191316.
5. Atun R, Davies JI, Gale EAM, Bärnighausen T, Beran D, Kengne AP, Levitt NS, Mangugu FW, Nyirenda MJ, et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol 2017; 5(8): 622–667.
6. Schaffert L-N, English E, Heneghan C, Price CP, Van den Bruel A, Plüddemann A. Point-of-care HbA1c tests – diagnosis of diabetes. Horizon Scan Report 0044. National Institute for Health Research 2016 (https://www.community.healthcare.mic.nihr.ac.uk/reports-and-resources/horizon-scanning-reports/point-of-care-hba1c-tests-diagnosis-of-diabetes).
7. Weykamp C, John G, Gillery P, English E, Ji L, Lenters-Westra E, Little RR, Roglic G, Sacks DB, et al. Investigation of 2 models to set and evaluate quality targets for HbA1c: biological variation and sigma-metrics. Clin Chem 2015; 61(5): 752–759.
8. Hirst JA, McLellan JH, Price CP, English E, Feakins BG, Stevens RJ, Farmer AJ. Performance of point-of-care HbA1c test devices: implications for use in clinical practice – a systematic review and meta-analysis. Clin Chem Lab Med 2017; 55(2): 167–180.
9. Lenters-Westra E, English E. Evaluation of four HbA1c point-of-care devices using international quality targets: are they fit for the purpose? J Diabetes Sci Technol 2018; 12(4): 762–770.
10. EurA1c Trial Group. EurA1c: the European HbA1c trial to investigate the performance of HbA1c assays in 2166 laboratories across 17 countries and 24 manufacturers by use of the IFCC model for quality targets. Clin Chem 2018; 64(8): 1183–1192.
11. Lenters-Westra E, Slingerland RJ. Three of 7 hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin Chem 2014; 60(8): 1062–1072.
12. Lenters-Westra E, English E. Understanding the use of sigma metrics in hemoglobin A1c analysis. Clin Lab Med 2017 Mar; 37(1): 57–71.
13. The use of POCT HbA1c devices in the NHS Diabetes Prevention Programme: recommendations from an expert working group commissioned by NHS England. NHS England Publications Gateway Reference 05139. NHS 2016 (https://www.england.nhs.uk/wp-content/uploads/2016/07/poct-paper.pdf)
The authors
Emma English*1 PhD, Larissa-Nele Schaffert2 BSc and Dr Erna Lenters-Westra3,4 PhD
1Faculty of Medicine and Health, University of East Anglia, Norwich Research Park, UK
2School of Medicine, University of Nottingham, Nottingham, UK
3Department of Clinical Chemistry, Isala, Zwolle, The Netherlands
4European Reference Laboratory for Glycohemoglobin, Location Isala, Zwolle, The Netherlands
*Corresponding author
E-mail: emma.english@uea.ac.uk