Point-of-care testing in the management of hemorrhage in cardiothoracic surgery
Cardiac surgery with cardiopulmonary bypass is associated with significant alterations in the hemostatic system. Standard laboratory coagulation tests are frequently used to assess coagulopathies and to guide hemostatic interventions. However, these tests present major limitations. Viscoelastic testing in conjunction with the implementation of a specific algorithm for coagulation management in cardiac surgery enables better control of perioperative coagulopathies.
Cardiac surgery and the hemostatic system
Cardiac surgery with cardiopulmonary bypass (CPB) is associated with significant alterations in the hemostatic system. Consequently, approximately 20% of patients present with postoperative bleeding. This causes high rates of surgical re-exploration due to bleeding (reaching 7% in valve surgeries), and high consumption of blood products, which results in high rates of morbidity and mortality associated with this type of intervention [1]. Adverse outcomes include blood-borne infections, transfusion reactions, respiratory dysfunction, increased cost, kidney failure, and increased mortality rates [2].
This review focuses on coagulopathies known to occur in cardiac surgery and addresses the monitoring of these hemostatic alterations.
Coagulopathies associated with cardiac surgery
The most important hemostatic alterations associated with CPB include the following.
Dilutional coagulopathy
Dilutional coagulopathy is mainly related to the priming of the CPB circuit and liberal crystalloid administration, and constitutes an important cause of coagulopathy in patients undergoing cardiac surgery. At the physiological level, it decreases circulating hemoglobin levels, reduces plasma concentrations of coagulation factors (factor VIII and von Willebrand factor), and causes thrombocytopenia and impaired platelet function.
Hypothermia
Hypothermia is induced intentionally to enable surgery during cardiac arrest. Hypothermia results in longer operative times, increasing the risk of infections and prolonging the contact time between the blood and the CPB circuit [3].
Acidosis
Acidosis is often induced by hypoperfusion and excess administration of NaCl during resuscitation and massive transfusion of blood products. Acidosis affects almost all phases of the coagulation process.
Consumption coagulopathy
During cardiac surgery with CPB, both the intrinsic pathway (through the activation of factor XII due to contact with the circuit surface) and the extrinsic pathway are activated (due to contact of the blood with the damaged tissues) [4].
Hyperfibrinolysis
Hyperfibrinolysis is a frequent complication of cardiac surgery that can lead to the development of microvascular bleeding [4].
Anticoagulation
Heparin (which considerably increases antithrombin activity) and protamine (used for heparin anticoagulation reversal) are administered during cardiac surgery. The use of heparin can cause hemorrhage due to the appearance of heparin-induced thrombocytopenia or due to an increase in heparin levels related with the rebound effect caused by the longer half-life of heparin compared to protamine [5].
These factors contribute to inflammatory and hemostasis dysfunction, which may lead to perioperative coagulopathy and postoperative bleeding.
Hemostasis monitoring in cardiac surgery
Perioperative management of coagulopathic bleeding requires timely hemostatic intervention using blood products. To guide these interventions, fast laboratory work-up is essential.
Standard laboratory coagulation tests [prothrombin time (PT)/international normalized ratio or partial thromboplastin time (aPTT), platelet count, and, in some cases, fibrinogen concentration] are frequently used to assess coagulopathy and to guide hemostatic interventions. However, this has been challenged in numerous reports, which question the utility of standard laboratory coagulation tests in the perioperative setting and in the event of massive bleeding [6]. Although they are frequently used to monitor perioperative coagulation, there is no robust evidence supporting their value for diagnosing coagulopathy during surgery or transfusion therapy, and their use seems to be based more on ‘habit’ than on scientific evidence.
These standard laboratory coagulation tests present major limitations. First, these tests are time-consuming (with turn-around times sometimes longer than 60 minutes); these times can be so long that results do not reflect the actual current clinical situation of the patient. Second, they do not allow for rapid evaluation in situations of severe bleeding that require immediate, targeted treatment [6,7]. In addition, standard laboratory coagulation tests only allow assessment of the initial phase of the coagulation cascade: because these classic tests are performed on plasma, they do not take into account the role played by platelets or other blood cells in the process. As a result, these tests do not provide information about the last phase of the process or fibrinolysis, and are unable to detect hyperfibrinolysis [7]. Third, there is no clinical correlation between results from these standard tests and the patient’s hemostasis status. No real correlation has been shown between the presence of excessive bleeding and prolonged aPTT and PT [8]. When these tests return abnormal results (prolonged clotting times), treatment options range from fresh frozen plasma to coagulation factor concentrates (such as factor VII or prothrombin complex concentrates). The administration of this type of product may be associated with the appearance of serious complications and, therefore, their administration must be rigorously justified [5]. Finally, in the case of platelets, these tests only provide quantitative information and do not inform about their functional capacity, and thus do not provide information about abnormalities acquired or induced during the procedure [7].
These standard laboratory coagulation tests are plasma-based tests that were essentially designed to monitor the action of vitamin K antagonists and heparin, and to assess coagulation factor deficiencies. The latter assays were not intended for monitoring of perioperative coagulation disorders, to predict bleeding, or to guide antihemorrhagic therapy in the perioperative setting [5].
Viscoelastic point-of-care testing (POCT) systems for coagulation monitoring are now available that may ultimately overcome several of the limitations associated with routine coagulation testing.
Viscoelastic testing: thomboelastography/thromboelastometry
Analytical POCT systems are currently available that enable coagulation monitoring and management of perioperative hemostasis disorders; these tests provide real knowledge of the patient’s coagulation status, enabling targeted hemostatic therapy.
Whole-blood viscoelastic tests [thomboelastography (TEG) and rotation thromboelastometry (ROTEM)] were developed by Hartert in 1948, and allow complete, dynamic measurement of the viscoelastic properties of blood with graphical representation of clot formation and the lysis process [9].
Both POCT technologies, TEG and ROTEM, are based on the same principle; although both terms are used interchangeably in the literature, there are subtle differences between them. In both tests, a citrate-anticoagulated blood sample is placed on a disposable cuvette (previously warmed to 37 °C) using an electronic pipette. A sensor or pin is placed in the sample and slowly rotated, generating a signal that is transmitted to a detector (in the case of TEG, the cuvette is rotated, rather than the pin). The method is based on the measurement and representation of the increasing resistance to which the pin is subjected as the coagulation process takes place. The assay begins with the addition of various reagents, and provides complete, detailed information on the processes of clot formation and lysis. Finally, the instrument graphically represents all stages of clot formation, together with a series of numerical parameters characterizing the hemostatic process, enabling the monitoring, diagnosis, and management of hemostasis disorders occurring during cardiac surgery [7].
Among the benefits of viscoelastic testing we would highlight the following.
- Timely results: TEG and ROTEM are POCT systems. This is the main advantage of viscoelastic tests over standard tests. TEG/ROTEM provide information about the patient’s hemostasis status in
a matter of minutes. Thus, the first results are available 5 or
10 minutes after the start of the test (clot amplitude at 5 minutes and at 10 minutes [A5 and A10, respectively]). - TEG/ROTEM provide a global characterization of the coagulation cascade, providing information on all phases of the hemostatic process, as these tests are performed on anticoagulated whole blood (not plasma) and, therefore, take into account the role played by platelets and other blood cells.
- TEG/ROTEM allow discrimination between the different pathophysiological situations that may be responsible for coagulation disorders.
- Viscoelastic whole-blood testing provides quantitative data on platelets and platelet functional capacity; therefore, these tests provide information about abnormalities acquired or induced during the surgical procedure.
- These tests are able to detect states of hyperfibrinolysis.
However, several concerns have also been raised regarding the use of viscoelastic POCT coagulation monitoring tests [10]. A major limitation of standard viscoelastic testing is its insensitivity to the effects of antiplatelet drugs.
Some studies suggest that the implementation of viscoelastic POCT monitoring, such as TEG and ROTEM, in conjunction with a specific algorithm for coagulation management in cardiac surgery, allows for better control of perioperative hemostasis disorders.
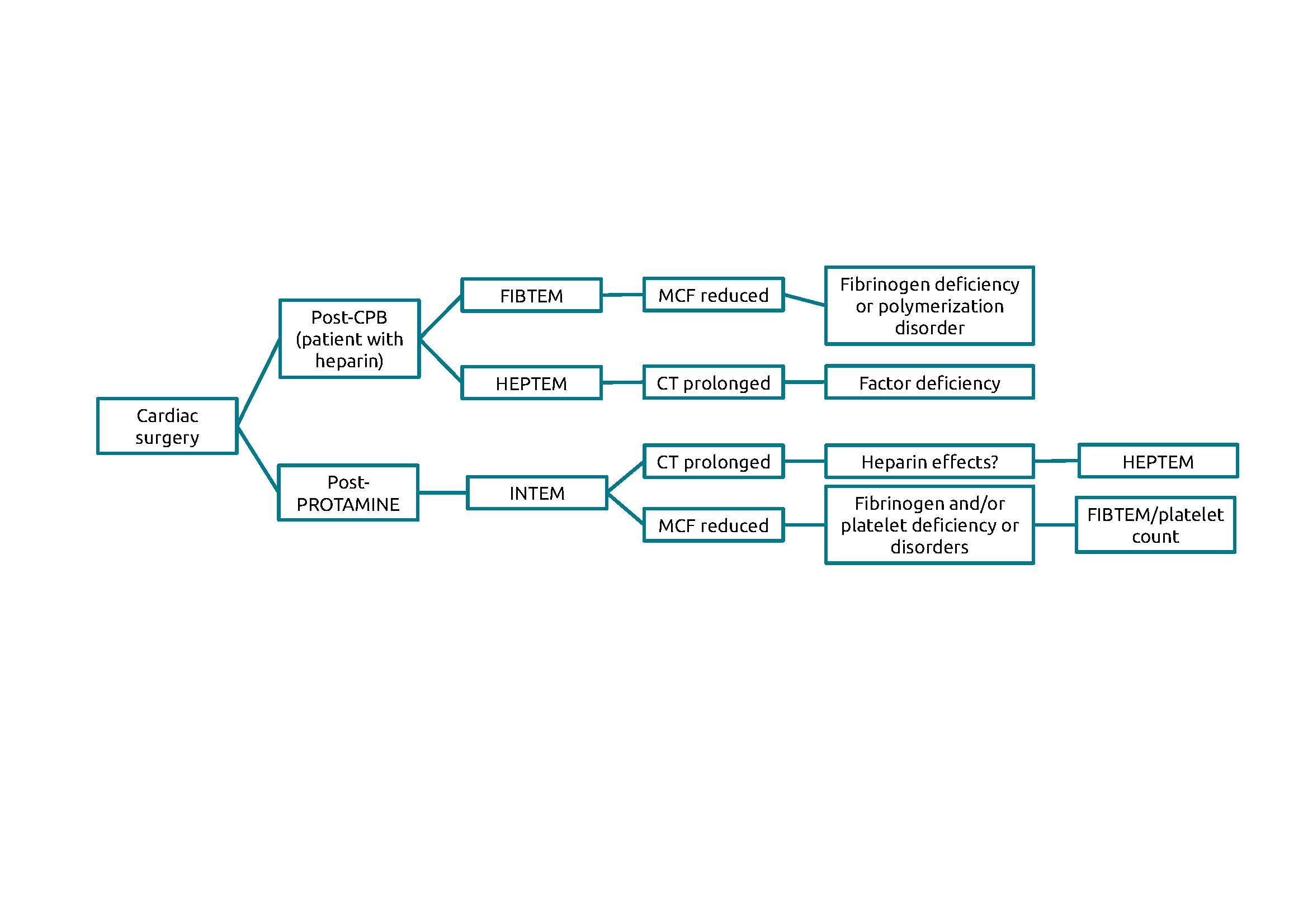
The specific algorithm for coagulation management was based on three tests: FIBTEM (which characterizes the contribution of fibrinogen levels to clot firmness), HEPTEM (which characterizes the intrinsic coagulation pathway when there are high levels of circulating heparin), and INTEM (which characterizes the intrinsic pathway following heparin neutralization with protamine).
The parameters recorded from these tests were: CT (time in seconds from addition of the start reagent to a clot firmness of 2 mm) in HEPTEM and INTEM, amplitude of clot firmness 10 minutes after CT (A10, mm), and maximum clot firmness (MCF, representing the greatest amplitude of the ROTEM® trace in millimetres) in FIBTEM, HEPTEM and INTEM [11].
The basic algorithm used for interpreting thrombobelastometry results (Fig. 1) [12] was conceived by the Essener Runde task force, which was created in 2001 and comprises specialists from different areas (anesthesia, intensive care, hematology, internal medicine, transfusion medicine, and surgery). The group is particularly interested in viscoelastic point-of-care tests, such as ROTEM [13].
Discussion: magnitude of the problem and whole-blood viscoelastic testing at a glance
Excessive peri-/postoperative bleeding is one of the most complex surgical complications due to its implications for short- and long-term morbidity and mortality. The management of this bleeding often requires re-exploration. Furthermore, patients undergoing reintervention present a postoperative progression characterized by a high rate of complications, greater consumption of blood products, longer ICU stays, and high mortality rates.
For all these reasons, it is essential to improve the monitoring and understanding of coagulopathies associated with cardiovascular surgery.
In this context, some studies suggest that the implementation of viscoelastic POCT monitoring, such as TEG and ROTEM, in conjunction with a specific algorithm for coagulation management in cardiac surgery, may allow for better control of hemostasis disorders. This targeted hemostatic therapy has been shown to be superior to empirical hemostatic therapy based on conventional laboratory coagulation tests and clinical judgment. Therefore, the use of viscoelastic testing, together with a specific algorithm for monitoring hemostasis, would appear to decrease transfusion requirements in cardiovascular surgery, associated morbidity, indirect hospital costs, and mortality [14,15].
Figure 1. ROTEM diagnostic algorithm of the ‘Essener Runde’ task force CPB, cardiopulmonary bypass; CT, time in seconds from addition of the start reagent to a clot firmness of 2 mm; FIBTEM, test that characterizes the contribution of fibrinogen levels to clot firmness; HEPTEM, test that characterizes the intrinsic coagulation pathway when there are high levels of circulating heparin; INTEM, characterizes the intrinsic pathway following heparin neutralization with protamine; MCF, maximum clot firmness. (Adapted from Lier H, Vorweg M, Hanke A, Görlinger K. Thromboelastometry guided therapy of severe bleeding. Essener Runde algorithm. Hamostaseologie 2013;33(1):51–61 [12])
The authors
Isabel Rodríguez-Martín1*, Catalina Sánchez-Mora2, Concepción González-Rodríguez2 and Víctor Sánchez-Margalet2
1 Clinical Biochemistry Department, Virgen del Rocío University Hospital, University of Seville, Seville, Spain
2 Clinical Biochemistry Department, Virgen Macarena University Hospital, University of Seville, Seville, Spain
*Corresponding author
E-mail: isarodm@gmail.com
References
- Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med 2004;30(10):1873–1881.
- Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood 1990;76(9):1680–1697
(https://doi.org/10.1182/blood.V76.9.1680.1680). - Ho KM, Tan JA. Benefits and risks of maintaining normothermia during cardiopulmonary bypass in adult cardiac surgery: a systematic review.
Cardiovasc Ther 2011;29(4):260–279 (https://doi.org/10.1111/j.1755-5922.2009.00114.x). - Johansson PI, Solbeck S, Genet G et al. Coagulopathy and hemostatic monitoring in cardiac surgery: an update. Scand Cardiovasc J 2012;46(4):194–202 (https://www.tandfonline.com/doi/full/10.3109/14017431.2012.671487).
- Singla A, Sullivan MJ, Lee G et al. Protamine-induced immune thrombocytopenia. Transfusion 2013;53(10):2158–2163
(https://onlinelibrary.wiley.com/doi/10.1111/trf.12112). - Hass T, Fries D, Tanaka KA et al. Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding:
is there any evidence? Br J Anaesth 2015;114(2):217–224 (https://doi.org/10.1093/bja/aeu303). - Whiting P, Maiwenn AI, Westwood M et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis:
a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19(58):1–228 (https://www.ncbi.nlm.nih.gov/books/NBK305697/). - MacLeod JB, Lynn M, McKenney MG et al. Early coagulopathy predicts mortality in trauma. J Trauma 2003;55(1):39–44
(https://journals.lww.com/jtrauma/Fulltext/2003/07000/Early_Coagulopathy_Predicts_Mortality_in_Trauma.7.aspx). - Galvez K, Cortes C. Tromboelastografıa: nuevos conceptos en la fisiologıa de la hemostasia y su correlacion con la coagulopatıa asociada al trauma [Thromboelastography: New concepts in haemostasis physiology and correlation with trauma associated coagulopathy]. Rev Colomb Anestesiol 2012;40(3):224–230 (in Spanish; http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-33472012000300011).
- Görlinger K, Tanaka KA, Dirkmann D. Whole blood assay: thromboelastometry. In: Teruya J (ed.) Management of bleeding patients, 1st edn;
Chapter 5, pp37–64. Springer 2016. ISBN 978-3319307244. - Görlinger K, Iqbal J, Dirkmann D, Tanaka KA. Whole blood assay: thromboelastometry – basics. In: Teruya J (ed.) Management of bleeding patients,
2nd edn; Chapter 6. Springer 2021. ISBN 978-3030563370. - Lier H, Vorweg M, Hanke A, Görlinger K. Thromboelastometry guided therapy of severe bleeding. Essener Runde algorithm. Hamostaseologie 2013;33(1):51–61.
- Karlsson M, Ternström L, Hyllner M et al. Plasma fibrinogen level, bleeding, and transfusion after on-pump coronary artery bypass grafting surgery:
a prospective observational study. Transfusion 2008;48(10):2152–2158 (https://doi.org/10.1111/j.1537-2995.2008.01827.x). - Görlinger K, Dirkmann D, Hanke AA et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology 2011 Dec;115(6):1179–1191 (https://doi.org/10.1097/ALN.0b013e31823497dd).
- Hanke AA, Herold U, Dirkmann D et al. Thromboelastometry based early goal-directed coagulation management reduces blood transfusion requirements, adverse events, and costs in acute Type A aortic dissection: a pilot study. Transfus Med Hemother 2012;39(2):121–128
(https://www.karger.com/Article/Pdf/337723).