Porphyrias: clinical and diagnostic aspects
Porphyrias are a group of disorders of the heme biosynthetic pathway which clinically manifest with acute neurovisceral attacks and cutaneous lesions. Diagnosis of porphyrias is based on the accurate and precise measurement of various porphyrins and precursor molecules in a range of samples. In addition, molecular diagnostic assays can provide definitive diagnosis.
by Dr Vivion E. F. Crowley, Nadia Brazil, and Sarah Savage
What are porphyrias?
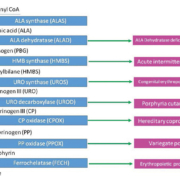
Porphyrias are a group of rare disorders each of which results from a deficiency of an individual enzyme within the heme biosynthetic pathway (Fig. 1) [1–3]. With the exception of an acquired form of porphyria cutanea tarda (PCT), all porphyrias are inherited as monogenic autosomal dominant, autosomal recessive or X-linked genetic disorders, with varying degrees of penetrance and expressivity and this impacts on the prevalence and incidence of clinically manifest porphyrias [4]. The biochemical consequence of each porphyria is the overproduction within the heme biosynthetic pathway of specific porphyrin intermediates and/or the porphyrin precursor molecules delta-aminolevulinic acid (ALA) and porphobilinogen (PBG) [2–3]. This in turn has implications for the clinical manifestation of these disorders, their overall classification and their diagnosis (see Table 2).
Clinical presentation
Porphyrias may present clinically with either or both of two symptom patterns. The first is the acute neurovisceral attack, which is a potentially life threatening episode related to excessive hepatic generation of ALA and PBG, and which is a feature only in acute intermittent porphyria (AIP), variegate porphyria (VP), hereditary coproporphyria (HCP) and the very rare ALA dehydratase deficiency porphyria (ADP) [5–7]. These attacks are characterized principally by autonomic dysfunction, including non-specific but severe abdominal pain, constipation, diarrhoea, nausea, vomiting, tachycardia, hypertension or occasionally postural hypotension. In addition, other features may include a predominantly motor peripheral neuropathy which, if left undiagnosed, may extend to respiratory failure reminiscent of Guillain–Barré syndrome, as well as cerebral dysfunction, which can vary from subtle alterations in mental state, to posterior reversible encephalopathy syndrome (PRES). Hyponatremia, most likely due to SIADH [syndrome of inappropriate antidiuretic hormone (ADH) secretion] may also contribute to CNS-related morbidity. The complex neuropathic manifestations appear to be primarily related to axonal degeneration due to direct neurotoxicity by ALA, which structurally resembles the neurotransmitter gamma-aminobutyric acid (GABA) [3, 5–7].
The second clinical presentation paradigm is cutaneous photosensitivity caused by the interaction of ultraviolet light with photoactive porphyrins in the skin resulting in the production of reactive oxygen species (ROS) and an associated inflammatory response [3]. In PCT, VP and HCP the skin lesions typically occur post-pubertally and consist of skin fragility, vesicles, bullae, hyperpigmentation and hypertrichosis affecting sun exposed areas, most usually the face and dorsum of hands [1–3]. In erythropoietic protoporphyria (EPP) and X-linked protoporphyria (XLP), which may present in childhood, there is usually no blistering but instead erythema, edema and purpura feature in the more acute setting, with subsequent chronic skin thickening noted, whereas congenital erythropoietic porphyria (CEP) is characterized by severe cutaneous photosensitivity often occurring in early infancy with bullae and vesicles rupturing and being prone to secondary infection, with resultant scaring, bone resorption, deformation and mutilation of sun-exposed skin [1, 2, 8].
Classification
The classification of porphyrias (Table 1) has traditionally been determined either on the basis of clinical manifestations, i.e. acute or non-acute (cutaneous), or on the primary organ of porphyrin overproduction, i.e. hepatic or erythropoietic [1, 3, 8]. A combined classification has recently been proposed which takes account of both of these elements [2]. However, whichever classification is adopted there should be a realization that VP, and to a lesser extent HCP, can manifest with both acute and cutaneous features either simultaneously or separately.
Clinical and biochemical diagnosis
The clinical manifestations of porphyrias, particularly the acute hepatic porphyrias, are protean and consequently, patients with a clinically active porphyria could initially present to a relatively wide spectrum of clinical specialties including, gastroenterology, acute medicine, dermatology, neurology, endocrinology and hematology amongst others [2]. In general, cutaneous porphyrias should not pose a diagnostic difficulty for an experienced dermatologist used to investigating photosensitive skin disorders, but biochemical testing is still required to define the type of porphyria present. However, definitive diagnosis of an initial acute hepatic porphyria attack is critically dependent on biochemical testing, as symptoms are often non-specific in nature (Tables 1 & 2).
The diagnosis of an acute hepatic porphyria attack is founded on demonstrating an increase in urine PBG levels in direct temporal association with the characteristic acute symptom complex, the minimum level of increase being between 2- and 5-fold [9, 10]. The urine PBG may be measured either as a random sample, where it should be reported as urine PBG to creatinine ratio or as a 24-hour urine collection, where total PBG is reported. The former has proven to be clinically efficacious and has the advantage of timeliness, reduced within-subject variation and convenience over the requirement for a 24 hour urine collection [9]. If the urine PBG is not elevated this effectively rules out an acute porphyria attack at the time of sampling, however, there are certain caveats to this. Thus it is important to note that if specific treatment with either heme preparations or carbohydrate loading has been instigated prior to the test these interventions could reduce the urine PBG level significantly, including normalization [3]. Furthermore, if the measurement of urine PBG is delayed or undertaken at a time removed from the actual acute clinical presentation e.g. by weeks or months, then the finding of a normal urine PBG at that later stage cannot effectively rule out acute porphyria [3]. In this authors experience another important caveat concerns patients with a previous confirmed diagnosis acute porphyria who present with symptoms suggestive of recurrent acute attack. In many instances these patients have a perpetually elevated urine PBG, even in between attacks, and therefore an elevated urine PBG cannot effectively guide diagnosis. In these situations a decision to treat as an acute attack has to be made on the basis of clinical findings.
Therefore, a clinically effective service for acute porphyria diagnosis requires that a timely, quality assured laboratory method for urine PBG should be available for analysis [11]. Although a qualitative method for urine PBG may suffice for the purposes of establishing a diagnosis this should be supported by the availability of a confirmatory quantitative method for urine PBG. The lack of availability of urine PBG assay is very often the basis for misdiagnosis or indeed delayed diagnosis of acute porphyria attacks [10].
In conjunction with PBG, urine ALA is often measured simultaneously and although also elevated it does not tend to reach the levels of PBG in acute porphyrias. The one exception is the extremely rare instance of autosomal recessive ADP due to defective ALA synthase 2 (ALAS2) activity, where markedly elevated urine ALA levels are reported while PBG may be normal or only slightly elevated [2, 3]. In addition, a similar pattern of urine ALA predominance relative to PBG (although not as elevated) may be observed in the context of lead poisoning, wherein patients may also present with abdominal pain and neuropathy [1, 3].
Once the diagnosis of acute porphyria has been made based on the urine PBG the next phase involves determining the type of porphyria present. This is very much dependent on the specific pattern of porphyrin overproduction observed in samples of urine, feces, plasma and erythrocytes. It is critically important that the laboratory analytical methods available extend beyond the sole measurement of total porphyrin levels [10–12]. In particular, it is essential that individual porphyrin analysis and isomer fractionation in both urine and feces is available to facilitate the identification of the porphyria-specific patterns of porphyrin overproduction [10–12]. In many instances non-porphyria disorders affecting the gastrointestinal and hepatobiliary systems or certain dietary factors may cause non-specific secondary elevations in porphyrins, e.g. coproporphyrinuria, which can be diagnostically misleading [3]. In such cases urine PBG levels will not be elevated and the pattern of porphyrins observed will not be indicative of any one of the specific porphyrias per se. Therefore, it is important to realize that a finding of elevated porphyrin levels does not automatically equate to a diagnosis of underlying porphyria. This further highlights the importance of developing specialist porphyria centres to ensure that the appropriate repertoire of quality assured testing and expert interpretation and support are available for diagnosis and management of porphyria patients [11, 13].
The diagnosis of cutaneous (non-acute) porphyrias is also very much based on the specific patterns of porphyrins observed in urine and feces. In addition, the pattern of free and zinc protoporphyrin in erythrocytes can be useful in the diagnosis of CEP, EPP and the related disorder, XLP. Moreover, the identification of the porphyria subtype, either acute or cutaneous, may also be enhanced by identifying characteristic plasma porphyrin fluorescence emission peaks, e.g. VP emission peak between 625 and 628 nm [1–3]. Finally, it is essential that all samples for porphyrin and precursor measurement are protected from light prior to analysis.
Role of genetic diagnosis
Given the heritable nature of porphyrias it is not surprising that molecular genetic analysis has also become an important diagnostic adjunct. There is an extensive allelic heterogeneity of pathogenic mutations among the implicated genes for each porphyria disorder, which means that most mutations are uniquely confined to one or at most a few kindreds. There are, however, a few exceptions to this trend, most notably in relation to founder mutations among the Swedish population and the Afrikaner population in South Africa. The general approach in the application of genetic diagnostic strategies is firstly to characterize the causative mutation in a known affected individual (proband) using a mutation scanning approach [14]. Once a putative mutation has been identified its pathogenicity for a particular porphyria should be affirmed and then more extensive family cascade genetic screening can be organized based on the analysis of this kindred-specific mutation [14].
This approach has important implications in the diagnosis of porphyria susceptibility, particularly for the autosomal dominant acute hepatic porphyrias, where both penetrance and expressivity of the disorders is low [3, 4]. Thus the penetrance among AIP, VP and HCP is between 10 and 40%, implying that the majority of patients with an autosomal dominant acute hepatic porphyria will not manifest with an acute attack (or indeed cutaneous lesions in the case of VP and HCP) in their lifetime [3, 4]. Moreover, this lack of penetrance may also extend to the absence of subclinical biochemical abnormalities indicative of an underlying autosomal dominant acute porphyria, demonstrating the limited sensitivity of biochemical testing in identifying asymptomatic family members.
Currently there is no clear-cut mechanism for discriminating between those who will manifest a clinical and/or biochemical phenotype and those who will not. While the role of environmental precipitating factors, e.g. porphyrinogenic medications, stress, prolonged fasting, menstruation [1–3], have long been recognized in triggering acute porphyria attacks, it is the presence of a pathogenic mutation which is still the single most important factor determining the overall susceptibility for an acute porphyria episode. Therefore, all patients carrying a pathogenic mutation should be regarded as pre-symptomatic carriers, i.e. capable of developing an acute attack, and one of the key applications of genetic analysis in the area is in identifying pre-symptomatic carriers to allow for appropriate counselling and management advice to prevent attacks [3, 14].
In this author’s experience another useful role for molecular diagnostics in porphyrias is in relation to those patients with an historic diagnosis of acute hepatic porphyria in whom the biochemical abnormalities have subsequently normalized over years. In such instances genetic analysis can provide a definitive diagnosis for the type of porphyria and will accommodate a more extensive family screening programme for potential pre-symptomatic carriers.
The current methods of genetic analysis vary but usually involve a confirmatory step using direct nucleotide sequencing of the putative pathogenic variants as the gold standard. However, the emergence of next generation sequencing platforms has further galvanized the diagnostic possibilities in this area. Overall, in autosomal dominant acute hepatic porphyrias, approximately 95% of mutations are identifiable [3, 14]. This sensitivity includes the application of additional methods such as ‘multiplex ligation-dependent probe amplification’ (MLPA) and gene dosage analysis for identifying complex mutations, such large gene deletions, which may not be detected using standard sequencing-based approaches [14].
In autosomal recessive porphyrias including ADP, CEP and EPP, the clinical penetrance approaches 100%. These disorders also display a level of genetic heterogeneity. In the case of EPP the presence of a relatively common low expression single nucleotide polymorphism (SNP) located in the ferrochetalase gene, FECH (IVS3-48C), appears to be essential for the clinical expression of the cutaneous phenotype in the vast majority of cases [15].
The application of molecular genetics has provided a means of establishing definitive porphyria susceptibility, however, similar to the situation for biochemical testing services any genetic diagnostic services in this area must be quality assured to a high standard and need to adopt appropriate mutation scanning assay validation protocols in accordance with international standards and best practice recommendations [11–14].
References
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet 2010; 375(9718): 924–937.
2. Balwani M, Desnick RJ. The Porphyrias: advances in diagnosis and treatment. Blood 2012; 120: 4496–4504.
3. Badminton MN, Elder GH. The porphyrias: inherited disorders of haem synthesis. In: Marshall W, Lapsley M, Day A, Ayling R, editors. Clinical Biochemistry Metabolic and Clinical Aspects. Churchill Livingstone Elsevier 2014; pp. 533–549.
4. Elder G, Harper P, Badminton M, Sandberg S, Deybach JC. The incidence of inherited porphyrias in Europe. J Inherit Metab Dis. 2013; 36: 849–857.
5. Simon NG, Herkes GK. The neurologic manifestations of the acute porphyrias. J Clin NeuroSci. 2011; 18: 1147–1153.
6. Sonderup MW, Hift RJ. The neurological manifestations of the acute porphyrias. S Afr Med J. 2014; 104: 285–286.
7. Crimlisk HL. The little imitator-porphyria: a neuropsychiatric disorder. J Neurol Neurosurg Psychiatry. 1997; 62: 319–328.
8. Siegesmund M, van Tuyll van Serooskerker AM, Poblete-Gutierrez P, Frank J. The acute hepatic porphyrias: Current status and future challenges. Best Pract Res Gastroenterol. 2010; 24: 593–605.
9. Aarsand AK, Petersen PH, Sandberg S. Estimation and application of biological variation of urinary delta-aminolevulinic acid and porphobilinogen in healthy individuals and in patients with acute intermittent porphyria. Clin Chem. 2006; 52: 650–656.
10. Kauppinen R, von und zu Fraunberg M. Molecular and biochemical studies of acute intermittent porphyria in 196 patients and their families. Clin Chem. 2002; 48: 1891–1900.
11. Aarsand AK, Villanger JH, Støle E, Deybach JC, Marsden J, To-Figueras J, Badminton M, Elder GH, Sandberg S. European specialist porphyria laboratories: diagnostic strategies, analytical quality, clinical interpretation and reporting as assessed by an external quality assurance programme. Clin Chem. 2011; 57: 1514–1523.
12. Whatley S, Mason N, Woolf J, Newcombe R, Elder G, Badminton M. Diagnostic strategies for autosomal dominant acute porphyrias: Retrospective analysis of 467 unrelated patients referred for mutational analysis of HMBS, CPOX or PPOX gene. Clin Chem. 2009; 55: 1406–1414.
13. Tollånes MC, Aarsand AK, Villanger JH, Støle E, Deybach JC, Marsden J, To-Figueras J, Sandberg S; European Porphyria Network (EPNET). Establishing a network of specialist porphyria centres – effects on diagnostic activities and services. Orphanet J Rare Dis. 2012; 7: 93.
14. Whatley SD, Badminton MN. The role of genetic testing in the management of patients with inherited porphyria and their families. Ann Clin Biochem. 2013; 50: 204–216.
15. Gouya L, Puy H, Robreau AM, Bourgeois M, Lamoril J, Da Silva V, Grandchamp B, Deybach JC. The penetrance of dominant erythropoietic protoporphyria is modulated by expression of wildtype FECH. Nat Genet. 2002; 30: 27–28.
The authors
Vivion E. F. Crowley*1 MB MSc FRCPath FFPath(RCPI) FRCPI, Nadia Brazil2 BA (Mod) FAMLS, Sarah Savage3 BSc MSc
1Consultant Chemical Pathologist, Head of Department, Biochemistry Department, St James’s Hospital, Dublin 8, Ireland
2Porphyrin Laboratory, Biochemistry Department, St James’s Hospital, Dublin 8, Ireland
3Molecular Diagnostic Laboratory, Biochemistry Department, St James’s Hospital, Dublin 8, Ireland
*Corresponding author
E-mail: vcrowley@stjames.ie