Potential breakthrough in early diagnosis of HPV16 cancers
The link between human papillomaviruses (HPVs) as the causal agents of anogenital warts and most incidences of cervical cancer has been longestablished, and it is in this background that the vaccine against HPV has been developed. However, more recently, HPV has also been found to be responsible for the increasing incidence and death from head, neck and anal cancer. Until recently, diagnosis has been challenging, usually involving invasive investigations, with patients presenting late. This article describes how testing for antibodies against the HPV L1 protein can be sensitive, specific, non-invasive and fast, which is an exciting development for the early diagnosis and therefore improved prognosis of patients with HPV-induced head, neck and anal cancers.
Background
Belonging to the taxonomic family Papillomaviridae, there are over 200 isolates (known as types) of human papillomaviruses (HPVs) which are transmitted through skin-to-skin contact and infect the basal cells of the skin or the mucous membranes [1]. Persistent infections with high-risk HPV types such as HPV16 can lead to the development of cancer of the infected epithelia, in particularly in the mouth, throat, cervix and anogenital region.
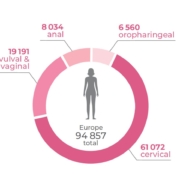
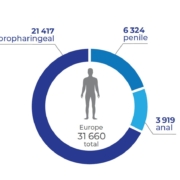
It is estimated that at least 80% of the population contract HPV at least once in their lives and most infections do clear within two years without symptoms occurring. However, over half of oropharyngeal and nine out of ten anal cancer cases are caused by high-risk HPV types, in particular HPV 16, which is responsible for up to 90% of all HPV-induced cases (Fig. 1) [2].
Worldwide, almost 700 000 people are diagnosed with an HPV-induced tumour every year [3]. Although cervical cancer still accounts for the largest proportion of cases, it is the continuing upward trend in developed countries of both incidence and death from head, neck and anal cancers that is now the cause of greatest concern (Fig. 2) [4–6].
Escalating number of preventable deaths
Data from cancer registries across Europe present a chilling picture when comparing outcomes of oral and pharyngeal squamous cell carcinomas with other skin cancers such as malignant melanoma. There are far more cases of malignant melanoma but, crucially, these are usually detected at an early enough stage to be treated successfully. For head and neck cancers, the pattern is completely different, with later diagnosis and mortality rates twice as high [7, 8].
Experts have been warning of a ‘virus-related cancer epidemic’ and calling for improved methods of early detection, treatment control and post-treatment surveillance [5]. What is especially disturbing is that we know that many of these deaths could be prevented with early detection methods that are accessible, affordable, and easily integrated into clinical practice.
Health service providers view HPV vaccination as the ultimate goal in HPV cancer prevention, with latest studies suggesting that it may also prevent anal as well as head and neck cancers caused by HPV. However, vaccine recommendations have only recently been introduced and are targeted at young individuals who will not reach cancer-relevant age for many decades. A This leaves an entire generation of unvaccinated adults at risk of developing HPV-induced cancers. Further, vaccination is currently only available to a small portion of the world’s population, with over seven billion individuals still unprotected [9]. HPV and HPVinduced cancers will therefore continue to be a challenge for many decades to come.
Limitation of current detection methods
The success of cervical cancer screening mainly relies on the cancerous cells being easy to locate and access so that the ‘Pap’ smear can be taken. This is not the case if the cancer is in the back of the mouth and throat, where most HPV-induced cancers are located. Germany, for instance, carries out visual screening of the mouth, throat and anogenital region as part of its national skin cancer prevention service. However, the detection of early-stage, HPV-induced lesions in this way remains challenging.
HPV-induced lesions often hide in the folds of the tonsils, making them very difficult to spot during any routine check-up. Although lesions could be detected using pharyngoscopy (or in the case of anal cancer, anoscopy) these procedures are expensive, timeconsuming and far too invasive to be used in routine screening. For this reason, around 70% of oropharyngeal cancers are usually only detected as late-stage tumours, increasing morbidity and reducing survival [10].
More recently, there has been a surge in the development of molecular methods for the detection of HPV DNA in swab material. It is important to note that these methods are very sensitive but cannot distinguish between simple HPV infections and HPV-induced tumours. This is demonstrated, for example, in an American study published in 2019, in which over 3000 healthy men were tested for oral HPV DNA. High-risk HPV was found in 7.3% of those examined – extrapolated to the male US population, this would correspond to about 12 million cases [11]. However, this compares to only about 16 000 oropharyngeal cancers actually diagnosed in 2020 [12].
If lives are to be saved, what emerges is a clear, threefold need: to (i) develop a non-invasive test that would be (ii) sensitive enough to detect HPV cancer but also (iii) specific enough to differentiate an HPV infection from actual HPV-induced malignancy – something that existing molecular tests are unable to do.
Pioneering immunological research
Recent research has taken a fundamentally different approach, moving beyond genotyping tests to look at the possibility of identifying an immune response elicited by HPV-induced tumour growth. Further, it has sought to address the need for a less invasive, easy-to-use, test that does not rely on taking cells samples from different parts of the body.
This has led to the development of a novel, blood-based HPV16 type-specific assay which relies on detecting an immune response – one that only occurs when an HPV infection begins to interfere with cell division, which is considered an early step towards tumour development [13–15]. The serological assay can be run as a lateral flow rapid test, delivering results in under 20 minutes, directly at the point of care.
Previously, no simple, blood-based assay has been available to detect HPV cancer or differentiate an HPV infection from HPV-induced malignancy or precancerous growth. Evidence from two recent clinical studies suggests this may now be possible [13–15], indicating that a breakthrough is on the horizon. These studies evaluated the performance of the blood-based assay (Abviris) with the potential to detect HPV16-induced cancer and predict its course from just a pinprick of blood [13–15].
HPV16 L1 – a long overlooked target
The assay is based on the highly specific detection of a patient’s immune response to the HPV16 L1 protein, a target previously overlooked because it was not thought to be expressed by an HPV-induced tumour. The HPV genome contains nine genes (E1–E7, as well as L1 and L2), with most studies focusing on oncogenes E6 and E7. Previously, it was believed that tumour cells did not produce L1 or that serum antibodies against L1 could discriminate between cancer and subclinical HPV infection. In spite of this, several historic studies had already contradicted this view, for example by showing that immune cells programmed to kill L1-positive cells were also able to destroy tumour cells [16–18].
In a multicentre European study published in EbioMedicine, Weiland et al. describe an immunostaining protocol where it was possible to visualize L1 expression in tumour tissue [13]. It shows that L1 is indeed expressed in HPV-induced oropharyngeal squamous cell carcinoma, raising the potential of using circulating antibodies against L1 as a blood-based tumour marker (Fig. 3).
The study, involving over 1000 blood samples from healthy individuals, established the specificity of the assay to be over 99%. It also examined samples from patients diagnosed with either HPV16-induced oropharyngeal or anal squamous cell carcinoma, demonstrating sensitivities for these cancers of 95% and 90%, respectively. In the anal cancer population, blood samples were available in the year (up to nine months) prior to cancer diagnosis. Remarkably, nine out of ten of these retrospective samples were found to be serologically positive, suggesting that these patients would have received an earlier cancer diagnosis if the rapid test had been available.
Biomarker tracking tumour development
In the second part of their study, Weiland and colleagues monitored HPV16 L1 antibody concentrations in patients with oropharyngeal cancer for a period of 24 months after treatment onset. The study team observed a decrease in antibody levels during successful treatment, whereas a rise during post-treatment follow-up was associated with the return of their cancer.
The protocol for the Weiland study now forms the basis of a separate and much larger investigation across at least 15 German hospital sites due to start later his year. Not only will the PRECISE study seek to confirm the ability of the assay to indicate if a tumour is caused by HPV – without the need for an invasive procedure like a biopsy – it will also look at the ability of the new blood-based assay to be used as a non-invasive tool in monitoring treatment success. At present, treatment success and cancer recurrence can only be monitored using high-cost imaging methods including PET-CT or invasive endoscopies performed under general anesthetic.
Together, these studies provide the first evidence that an easy-to-use, blood-based test is able to closely track HPV tumour develop-ment. Given the low rates of positive results among the general population, combined with high sensitivity observed in target patient groups, the results of the Weiland study support the view that the assay would be able to detect HPV-induced malignancies, without identifying transient, subclinical HPV infections.
Impact on patient outcomes
There is a broad range of potential applications for the novel Abviris biomarker capable of tracking HPV tumour development. The most immediate application lies in the post-treatment monitoring of patients treated for HPV-induced cancers. A blood-based biomarker test has the potential to reduce the frequency of invasive procedures as well as the demand for imaging, allowing highercost resources to be used in a more targeted manner. Identification of rising antibody levels could be used to flag suspected cases of tumour recurrence, leading to prioritization of appointments and earlier intervention.
A further potential application lies in using the format of a rapid antibody assay as a simple screening tool for asymptomatic individuals, aiming to catch more cancer cases at an earlier stage. The way this would be implemented might vary according to each country’s medical system and health care needs. In Germany, where there is already a national skin cancer screening programme, some clinicians have already started piloting the Abviris point-of-care test as an additional service. Another example would be to integrate it into any existing oral examination during routine dental check-ups.
Alternatively, the test could be used in a more targeted way, aimed at patients at greatest risk, such as those with recurring genital warts, multiple sexually transmitted infections and/or HIV, and those engaging in anal sex. Testing could be offered at sexual health clinics, and those with a positive result fast-tracked for further investigation.
The new serological HPV tumour marker could not only boost HPV cancer prevention by catching more cases at an earlier stage, but significantly improve treatment for those where prevention comes too late. Overall, this would significantly reduce mortality and increase the quality of life for patients of this rapidly spreading HPV cancer epidemic.
The author
Anna Huber PhD
Abviris GmbH, 22926 Ahrensburg, Germany
E-mail: anna.huber@abviris.com
References
1. De Villiers EM, Fauquet C, Broker TR, Bernard HU, Zur Hausen H. Classification of papillomaviruses. Virology 2004; 324(1): 17–27 (https://bit.ly/2XCaUE8).
2. Bruni L, Albero G, Serrano, Mena M, Gómez D, et al. Human papillomavirus and related diseases: Germany. ICO/IARC Information Centre on HPV and Cancer
(HPV Information Centre) 2019 (https://hpvcentre.net/statistics/reports/DEU.pdf?t=1575294458729).
3. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob
Health 2020; 8(2): e180–e190 (https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(19)30488-7/fulltext).
4. Deshmukh AA, Suk R, Shiels MS, Sonawane K, Nyitray AG, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United
States, 2001-2015. J Natl Cancer Inst 2020; 112(8): 829–838 (https://academic.oup.com/jnci/article/112/8/829/5622917).
5. Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010; 11(8): 781–789
(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5242182/).
6. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States.
J Clin Oncol 2011; 29(32): 4294–4301 (https://bit.ly/3C6hGAT).
7. Kaatsch P, Spix C, Katalinic A, Hentschel S, Luttmann S, et al. Krebs in Deutschland für 2013/2014. Robert Koch Institute 2017 (in German; https://bit.ly/3DSiJVE).
8. Head and neck cancer statistics: incidence and mortality
(https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers) and Melanoma skin cancer
statistics (https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/melanoma-skin-cancer). Cancer Research UK.
9. Monitoring and Surveillance of HPV Vaccination Programmes. World Health Organization (WHO) 2020 (https://www.who.int/teams/immunization-vaccinesand-
biologicals/diseases/human-papillomavirus-vaccines-(HPV)/hpv-clearing-house/monitoring).
10. Wienecke A, Kraywinkel K. Epidemiology of head and neck cancer in Germany. Onkologe 2019; 25(3): 190–200 (in German).
11. Bettampadi D, Villa LL, Ponce EL, Salmeron J, Sirak BA, et al. Oral human papillomavirus prevalence and type distribution by country (Brazil, Mexico and the United
States) and age among HPV infection in men study participants. Int J Cancer 2020; 146(11): 3026–3033 (https://onlinelibrary.wiley.com/doi/10.1002/ijc.32713).
12. Cancers associated with human papillomavirus, United States, 2013–2017. United States Cancer Statistics Data Brief, No 18. Centers for Disease Control and
Prevention, US Department of Health and Human Services 2020; 1–2 (https://www.cdc.gov/cancer/uscs/pdf/USCS-DataBrief-No18-September2020-h.pdf).
13. Weiland T, Eckert A, Tomazic PV, Wolf A, Pondorfer P, et al. DRH1 – a novel blood-based HPV tumour marker. EBioMedicine 2020; 56: 102804
(https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(20)30179-1/fulltext).
14. Blatt S, Pabst A, Zimmer S, Walter C, Al-Nawas B, Krüger M. Clinical efficacy of an antibody-based detection system for human papilloma virus infection in oral
squamous cell carcinoma. Clin Oral Investig 2021; 25(5): 2837–2843 (https://link.springer.com/article/10.1007%2Fs00784-020-03601-0).
15. Ecke S, Huber A, Hilfrich R, French LE, Reinholz M. Serological HPV tumor maker DRH1 shows high sensitivity and specificity for anal dysplasia in people living
with HIV. Submitted for publication 2021.
16. Bellone S, El-Sahwi K, Cocco E, Casagrande F, Cargnelutti M, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T
lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for
L1 dendritic cell-based therapeutic vaccines. J Virol 2009; 83(13): 6779–6789 (https://bit.ly/3DVHwIc).
17. De Bruijn ML, Greenstone HL, Vermeulen H, Melief CJ, Lowy DR, et al. L1-specific protection from tumor challenge elicited by HPV16 virus-like particles.
Virology 1998; 250(2): 371–376 (https://reader.elsevier.com/reader/sd/pii/S0042682298993722?token=9D041CC6E655DF433419715B81EE37B75E32A3C86
56AA7D76EE6A7DAA8A19AC15E3DA300671DC5045321FC0A9FFFAD94&originRegion=eu-west-1&originCreation=20211030141503).
18. Luxton JC, Shepherd PS, Coletart T, Rose RC, Wilson P. Serological and T-helper cell responses to human papillomavirus type 16 L1 in women with cervical
dysplasia or cervical carcinoma and in healthy controls. Journal of General Virology 1997; 78(4): 917–923
https://www.microbiologyresearch.org/content/journal/jgv/10.1099/0022-1317-78-4-917#tab2
19. International Agency for Research on Cancer. Cancer today: Estimated number of new cases in 2020, Europe, both sexes, all ages. World Health Organization
(https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_
population=continents&population=900&populations=908&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_
group%5B%5D=0&ages_group%5B%5D=17&group_cancer=0&include_nmsc=0&include_nmsc_other=1#collapse-group-1-4-0).