Progress in biomarkers of colorectal cancer
Colorectal cancer is one of the commonest types of cancer and contributes significantly to cancer-related mortality. Recent research has focused on the identification, development, validation of sensitive and specific biomarkers to improve early diagnosis, to assess disease outcome, accurately predict response to therapy or monitor disease status after treatment.
by Dr Caroline Coghlin and Professor Graeme I Murray
The requirement for biomarkers in colorectal cancer
Colorectal cancer (CRC) represents a heterogeneous disease with variable clinical presentations and equally variable outcomes observed between individual patients. At the molecular level, although several well-described pathways are known to exist in CRC tumorigenesis the reality is likely to be much more complex. In addition to genetic alterations, numerous interactions exist between multiple abnormal signalling networks and between the tumour cells and their microenvironment. Surgery is the mainstay of treatment in primary CRC but options for adjuvant or neoadjuvant chemotherapy are advancing. Given these choices, and the potential toxicity associated with some agents used, the need for validated and reliable biomarkers to aid in CRC diagnosis, management and predictive and prognostic stratification is growing [1].
Diagnostic, prognostic and predictive biomarkers
The detection of protein biomarkers using immunohistochemistry in fixed tissue is a reliable technique widely used in the histopathology laboratory setting. Accurate diagnosis of primary CRC is often clear from its site of origin detected colonoscopically or with imaging modalities and from the morphological features revealed on histology. However, diagnosis of CRC in secondary sites can be more difficult. CRC cells frequently express CK20 and CDX2 (an intestinal-specific transcription factor) and they are generally negative for CK7. Therefore, a targeted panel of immunohistochemical markers can aid in the diagnosis of metastatic CRC and so guide appropriate treatment [2].
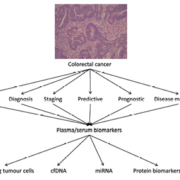
Accurate prognosis in CRC currently relies on pathological and clinical staging including use of the tumour, node, metastasis (TNM) system. In the past, assay of plasma biomarkers such as carcinoembryonic antigen (CEA) and CA19.9 to aid in prognostic stratification was advocated but, while these markers may still have a role in disease monitoring after surgery, as diagnostic and prognostic tools such biomarkers lack sensitivity and specificity. A large body of research has focused on comparative proteomic analysis of primary site CRC, normal control tissue and metastatic tissue in attempts to identify clinically useful prognostic and predictive CRC biomarkers [3, 4]. Candidate markers have included the mismatch repair proteins, especially MLH1 and MSH2 (which may be linked with a better prognosis in early CRC but also with a poor response to some chemotherapeutic agents such, as 5-fluorouracil) along with many other molecules involved in diverse cellular processes such as matrix degradation, oxidative metabolism or protein folding [3, 4]. There is, however, often a deficiency in the consistent follow-up studies necessary for the validation of such potential biomarkers. This aspect of study remains crucial to enable the robust and reliable clinical application of biomarkers in CRC [Fig. 1].
Plasma/serum biomarkers in CRC
The goal of screening programmes for CRC is to decrease mortality and reduce morbidity associated with the disease by identifying cancers at an early stage when intervention is more likely to succeed. Fecal occult blood or DNA testing and targeted colonoscopy are methods commonly employed to screen for early lesions. Fecal testing has the disadvantage of poor patient acceptability and it also lacks sensitivity. Colonoscopy, while accurate, is invasive and associated with a small but significant risk to the patient. With this in mind, the ultimate aim of many recent studies has been to identify robust plasma-based biomarkers which represent an acceptable form of patient testing. Such biomarkers should ideally be able to identify early invasive or perhaps even pre-invasive CRC in a sensitive and specific manner.
Circulating tumour cells (CTCs) are malignant cells originating from a primary tumour or its metastases which have gained access to the bloodstream. Several methods have recently been developed to identify these cells which may be associated with a poorer prognosis or an increased risk of relapse after surgery. In addition, molecular genetic characterisation of CTCs has potential implications for targeted therapy and therefore predictive value. CTCs in CRC patients are more abundant in central blood compartments such as the mesenteric vessels but peripheral blood can also be a source providing enrichment techniques are employed. In patients with metastatic CRC identification of CTCs in peripheral blood is associated with an adverse prognosis [5]. In addition, post-operative measurement of CTC levels may be used to predict tumour recurrence after surgery [6]. However, attempts to link CTC levels in early stage CRC (stage 1 disease) with an adverse prognosis have shown less convincing results and therefore the utility of this method as a screening modality is currently unclear [7].
Increased levels of circulating cell-free DNA (cfDNA) in cancer patients have been studied as a potential biomarker of the disease [8]. The precise source of these cfDNA fragments in conditions such as CRC remains controversial but tumour cell apoptosis, necrosis, or possibly active secretion have been suggested as putative mechanisms. A recent study used quantitative real-time PCR to detect ALU repeats in the plasma or serum of operated or non-operated CRC patients and compared the levels of circulating cfDNA detected with normal healthy controls [9]. The results showed that circulating levels of cfDNA were significantly higher in non-operated CRC patients when compared with operated patients and controls. This study concluded that quantification of serum cfDNA could be important in detecting and monitoring CRC patients in both early and late stage disease. The predictive value of cfDNA analysis has also been suggested. A subset of patients with initial wild type KRAS status receiving monotherapy with epidermal growth factor receptor (EGFR) inhibitors have been shown to develop early KRAS mutations detectable in cfDNA in the serum months before imaging revealed disease progression [10]. Despite progress in the detection and monitoring of cfDNA there are no reproducible studies to date to show direct and consistent correlation between CRC stage and circulating cfDNA levels. As laboratories use different methods to detect, analyse and monitor cfDNA, future translation to clinical use will require further standardization to ensure consistency in analysis.
MicroRNAs (miRNAs) are small non-coding RNA strands that can post-transcriptionally regulate the expression of multiple target genes. They have been implicated in several steps in carcinogenesis and their measurement in the serum of CRC patients has shown early promise for disease detection and monitoring of cancer recurrence after surgery [8]. miRNA-29c is thought to suppress tumorigenesis by inhibiting cell proliferation and migration. Circulating miRNA-29c levels have been studied as a potential biomarker for both early and late recurrence following surgery in colorectal cancer [11]. The oncogenic miRNA, miRNA-21, which negatively regulates tumour suppressor genes, has shown promise as a possible diagnostic and prognostic marker in CRC. Levels of this miRNA were significantly raised in CRC patients and patients with colonic adenomas when compared with healthy controls [12]. Serum miRNA-21 levels also fell in those patients undergoing curative surgery. Increased levels of this miRNA in both tumour tissue and the patients’ serum were found to be significantly associated with tumour size, the presence of metastasis and reduced survival. A possible drawback of miRNA analysis in cancer patients is the variable extraction rates of these small molecules from patients’ plasma or serum. To date this has produced inconsistent results between different studies. Once again a standardized approach will be required.
Proteomic analysis of tissue samples in CRC has been proven a valuable technique for the identification of potential biomarkers. Given the issues surrounding patient acceptability with the use of faecal material or invasive techniques in CRC screening, recent studies have focused on identifying plasma or serum protein biomarkers which can aid in the early diagnosis of CRC. Choi et al. analysed plasma samples from patients with colorectal adenomas or invasive disease and identified a panel of proteins, including three cytokines, which were differentially expressed between the study groups [13]. Another group used combinations of serum CEA, cytokines and CA19.9 to try to differentiate adenoma-bearing patients from healthy controls or those with established CRC [14]. Multiplex protein arrays have been developed to analyse serum samples from CRC patients, adenoma-bearing patients and healthy controls. Initial results indicated that combinations of CEA and IL-8 or CEA and C-reactive protein showed the best screening performance for early CRC or adenoma detection [15]. However, although the overall specificity of the tests employed was relatively high, the sensitivity was much lower (particularly with regard to adenoma detection) and the authors concluded that clinical use of such novel systems could be made in combination with established techniques such as faecal testing, for screening purposes.
Conclusions
Although significant progress has been made recently in the development of biomarkers in CRC there is still a relative lack of consistent follow-up data available for the validation of such markers. This aspect of research needs to be addressed in order to facilitate the transition of putative biomarkers from the research stage into robust and reliable clinical applications. The search for acceptable plasma-based biomarkers to aid in screening is gaining momentum but perhaps in future a combination of techniques will be required to accurately guide early diagnosis and intervention in colorectal cancer.
References
1. De Wit M, Fijneman RJ, Verheul HM, et al. Proteomics in colorectal cancer translational research: biomarker discovery for clinical applications. Clin Biochem. 2013; 46: 466–479.
2. Coghlin C, Murray GI. Following the protein biomarker trail to colorectal cancer. Colorectal Cancer 2012; 1: 93–96.
3. Ralton LD, Murray GI. Biomarkers for colorectal cancer: identification through proteomics Curr Proteomics 2010; 7: 212–221.
4. O’Dwyer D, Ralton LD, O’Shea A, Murray GI. The proteomics of colorectal cancer: identification of a protein signature associated with prognosis. PLoS One 2011; 6: e27718.
5. Groot Koerkamp B, Rahbari NN, Büchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic molorectal mancer: a meta-analysis. Ann Surg Oncol. 2013; 20: 2156–2165.
6. Galizia G, Gemei M, Orditura M, et al. Postoperative detection of circulating tumor cells predicts tumor recurrence in colorectal cancer patients. J Gastointest Surg. 2013; Epub ahead of print, doi: 10.1007/s11605-013-2258-6.
7. Linuma H, Watanabe T, Mimori K et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol. 2011; 29: 1547–1555.
8. Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11: 426–437.
9. da Silva Filho BF, Gurgel AP, Neto MÁ, et al. Circulating cell-free DNA in serum as a biomarker of colorectal cancer. J Clin Pathol. 2013; 66: 775–778.
10. Misale S, Yaeger R, Hobor S et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012; 486: 532–536.
11. Yang I-P, Tsai H-L, Huang C-W, et al. The functional significance of microRNA-29c in patients with colorectal cancer: A potential circulating biomarker for predicting early relapse. PLoS One 2013; 8: e66842.
12. Toiyama Y, Takahashi M, Hur K, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst. 2013; 105: 849–859.
13. Choi JW, Liu H, Shin D H, et al. Proteomic and cytokine plasma biomarkers for predicting progression from colorectal adenoma to carcinoma in human patients. Proteomics 2013; 13: 2361–2374.
14. Pengjun Z, Xinyu W, Feng G, et al. Multiplexed cytokine profiling of serum for detection of colorectal cancer. Future Oncol. 2013; 9: 1017–1027.
15. Bünger S, Haug U, Kelly M, et al. A novel multiplex-protein array for serum diagnostics of colon cancer: a case-control study. BMC Cancer 2012; 12: 393. doi: 10.1186/1471-2407-12-393.
The authors
Caroline Coghlin1 BA, BM Bch, DRCOphth, FRCPath; Graeme I. Murray2* MB ChB, PhD, DSc, FRCPath
1Department of Pathology, Aberdeen Royal Infirmary, NHS Grampian, Aberdeen, UK
2Pathology, Division of Applied Medicine, School of Medicine and Dentistry, University of Aberdeen, Aberdeen, UK
*Corresponding author
E-mail: g.i.murray@abdn.ac.uk