Q² Solutions unveils innovative self-collection safety lab panel in collaboration with Tasso
Q2 Solutions, a wholly owned subsidiary of IQVIA and a leading global clinical trial laboratory services organization, has launched the first self-collection safety lab panel for U.S. clinical trial participants by a leading global clinical trial laboratory.
Developed in collaboration with Tasso Inc, a leader in clinical-grade blood collection solutions, this unique offering combines industry leading laboratory services, patient focused logistics and cutting-edge, self-collection technology.
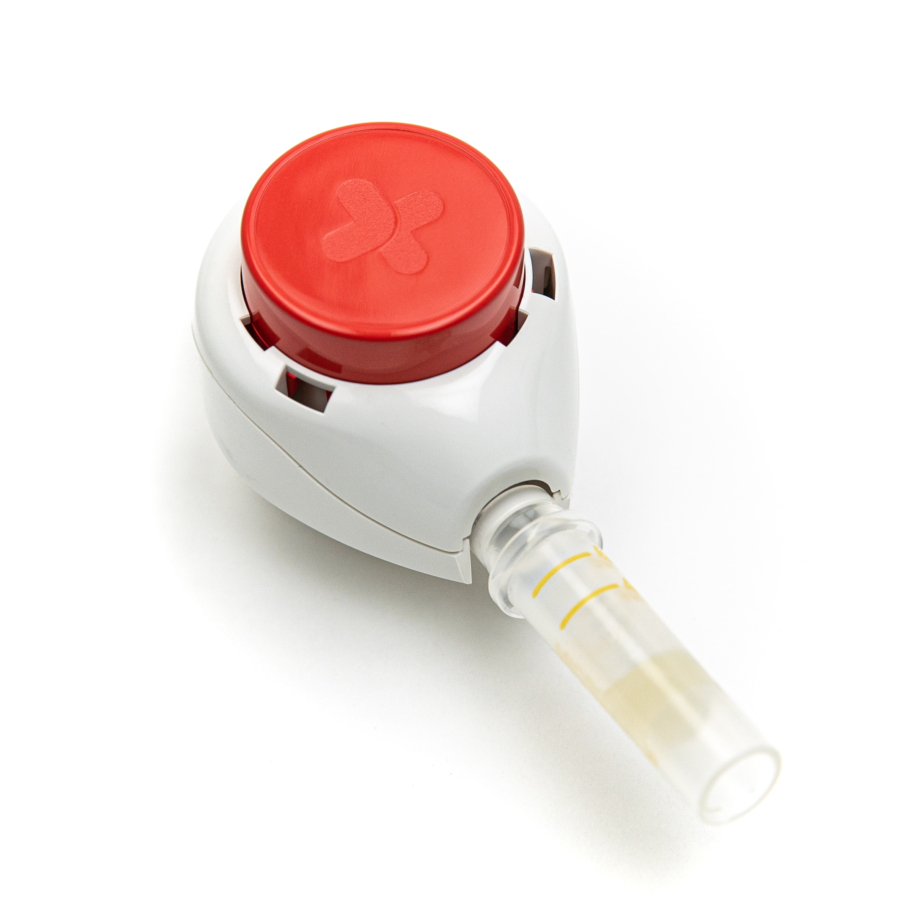
Participants in clinical trials can now provide a blood specimen for lab testing in the comfort of their own home, without the need to visit an investigator site or have a healthcare professional visit them. The first test panel available through this collaboration is designed to help monitor liver function by measuring the levels of select enzymes and proteins in the blood. The panel includes tests for ALT (alanine transaminase), AST (aspartate aminotransferase), ALP (alkaline phosphatase), total bilirubin, direct bilirubin, total protein, and albumin. “The global pandemic has accelerated the use of decentralized approaches in clinical trials,” said Brian O’Dwyer, CEO of Q2 Solutions. “We at Q2 Solutions are highly motivated to find ways to reduce patient burden related to laboratory testing, and I’m delighted that, through our collaboration with Tasso, we could make the self-collection liver panel a reality. Soon we will launch more than a dozen safety tests, and we are determined to continue to expand our catalogue of tests available via the self-collection of blood.”
“We are thrilled to be working with a leader like Q2 Solutions on this important initiative to make laboratory testing truly patient-centric,” said Erwin Berthier, PhD, CTO and Co-Founder of Tasso. “Our clinical-grade remote sampling technology enables decentralized laboratory testing without sacrificing sample quality, a key hurdle that home testing has faced in the past. Together, Q2 Solutions and Tasso are developing important solutions that make clinical trials better for both patients and sponsors.”
This self-collection liver panel was developed with trial participants at the heart of the design. The offering includes easy to use at-home self-collection kits with instructions and videos, a methodology for direct-to-patient delivery, as well as simplified specimen processing and shipping requirements
For more information, visit: www.q2labsolutions.com