Quality control testing on a random access molecular diagnostics platform running quantitative viral load assays
The DxN VERIS Molecular Diagnostics System* from Beckman Coulter is a real-time PCR analyser for accurate and precise quantitative detection of both RNA and DNA targets. Single sample random access offers workflow flexibility and automation benefits to the laboratory. The design features of the DxN VERIS System and performance characteristics of the VERIS HCV, HIV-1, HBV, and CMV viral load assays enable laboratories to develop Quality Control (QC) programmes tailored to their unique needs. Methods: A QC programme was developed by the Virology lab at the Rennes University Hospital, France. The laboratory evaluated the performance levels of the DxN VERIS System as well as the total number of VERIS HIV-1, HBV, and CMV tests performed over a period of five months. Results: The precision observed over the five-month study period was less than 5.8% CV with standard deviation (SD) within 0.16 log IU/ml. Based on these results the laboratory concluded that performing three levels of QC (negative, low, high) two times per week would provide an acceptable level of system control while significantly reducing QC costs and hands-on time.
Introduction
Consistency in reporting quantitative viral load results is critically important to clinical laboratories, physicians, and the patients they serve. The use of quantitative tests to measure viral load levels in patient samples is especially important for monitoring treatment. With the advent of new quantitative PCR (qPCR) assays for viral load testing, physicians are better able to manage diseases with antiretroviral therapy (ART).
Clinical laboratories are challenged to achieve stringent Quality Control (QC) objectives for viral load testing in an effective and economical manner. The use of external quality controls (EQC) provides laboratories with a means of monitoring variation in the analytical process as well as environmental factors that can affect patient results. In addition, EQC can assist laboratories in identifying when errors are occurring that can impact the utility of viral load assays. For these reasons, manufacturers of qPCR systems may recommend the use of EQC as part of the analytical process for viral load testing.
The DxN VERIS System and VERIS viral load assays are designed to deliver a high standard of clinical performance while providing rapid, convenient, and cost effective QC alternatives to the laboratory.
Quality control for quantitative diagnostic systems – a statistical approach
Statistical QC is defined as a procedure in which stable samples are measured and the observed results compared with limits that describe the variation expected when the measurement method is working properly[2]. Statistical QC is important to ensure the quality of the test results produced by any measurement method. An important concept in statistical QC is the definition of an “analytical run”. With many modern analytical systems, the definition of a run is not always clear. For example, many molecular diagnostics analysers available to laboratories today perform testing in “batch” mode, wherein each run corresponds to a single batch of several tests. While these methods can provide efficiencies in some testing environments (e.g. high volume labs) they can result in delayed results while the laboratory waits to accrue sufficient samples to complete the batch. In addition, batch systems lack the flexibility to adapt to fluctuating testing demand driven by sample volume and clinical needs in the laboratory. New qPCR systems are now available that provide “random access” capability; enabling labs to test individual samples at the precise time that they are most needed. In addition to providing more timely results for physicians and patients, these systems can also increase laboratory work flow efficiency, resulting in less hands-on time. For random access systems, an analytical run can be better understood in terms of the time or number of measurements for which the measurement is stable[2]. Statistical guidance for molecular assays typically suggests that quality control samples should be run at least once during each user-defined analytical run.
The DxN VERIS system
The DxN VERIS System is a fully automated molecular diagnostic system that integrates nucleic acid extraction, reaction setup, real-time PCR amplification and detection, and results interpretation into one system; saving space and time. The system provides single sample random access capability which allows the laboratory to run the right viral load test at the right time for physicians and patients. The DxN VERIS System provides time and workflow advantages compared to batch systems which require the laboratory to accrue a number of patient samples prior to each run.
Designed for quality and accuracy
The DxN VERIS System is engineered to deliver a high level of reliability and process control. The system provides a comprehensive range of individual process checks throughout the analytical process, from sample introduction to result reporting. Listed below are key features of the DxN VERIS System that ensure consistent performance and process control. Collectively, these capabilities may serve to reduce risk of analytical error within run and between runs.
Sample introduction
- Sample obstruction detection
- Liquid level sensing of sample and key reagents
- Fully automated end to end processing, which minimizes sample handling
Nucleic acid extraction
- Unitized extraction and purification (EP) cartridges to minimize contamination risk
- Internal thermal control to enable repeatable clinical results across the specified range of laboratory conditions
- Same hardware same pathway for extraction of all samples (transfers, pumps, motors)
- Dedicated pipette tip for each reagent to minimize risk of contamination
Real-time PCR amplification and detection
- Precise thermal control of PCR vessel during PCR processing
- One-wire chip located in each assay reagent pack (ARP) to ensure reagent life and calibration
- Uracil-DNA-glycosylase (UDG) enzyme in the PCR reaction mix. UDG digests uracil-containing amplicons created in previous PCR reactions
An internal process control (PC) is run with each sample to monitor the reaction. The PC may be a plasmid or an inactivated virus that contains a selected or engineered target sequence and is designed to mimic the behaviour of the assay target throughout the extraction, purification, and PCR process.
In addition to these quality features, the DxN VERIS System displays QC results in chart format to provide a graphical view of the data. Depending on the characteristics of the data, the system uses a Levy-Jennings chart or a Shewhart chart. Multiple data sets can be viewed simultaneously in an overlay chart, or in up to four individual charts. On- board QC management software flags when QC is out of range.
QC procedure for VERIS viral load assays
Beckman-Coulter’s QC procedure provides a method of monitoring system performance while minimizing hands-on time and QC cost to the laboratory. Beckman Coulter recommends that Quality Control should be run in each 24-hour period in which test samples are run until variability limits have been established on the DxN VERIS System. Reduced frequency of control testing should be based on data as determined by the individual laboratory. Quality control materials should incorporate the analyte and a negative control.
Each laboratory should establish mean values and acceptable ranges to assure proper performance. Quality control results that do not fall within acceptable ranges may indicate invalid test results. It is recommended that laboratories examine all test results generated after obtaining the last acceptable quality control test point for this analyte.
In some countries or geographic locations, government regulation may define specific requirements that dictate frequency and number of QC data points and specimens used. Each laboratory should establish its own QC protocol based on data as determined by the laboratory in accordance with accrediting organizations and government regulations, as applicable[2].
Customer case study – Rennes University Hospital
Quality Control programmes utilizing the DxN VERIS System have been successfully implemented at customer laboratories across Europe. Described below is an example of a QC protocol developed in the Virology laboratory at Rennes University Hospital, France.
Rennes University Hospital is a 2,000 bed facility serving the Brittany region of France. The Virology lab processes approximately 133,000 analyses per year including 8,000 qPCR tests for HIV-1, HBV, and CMV viral load monitoring. The laboratory adopted the DxN VERIS system in 2016 based on the system’s workflow advantages and assay performance.
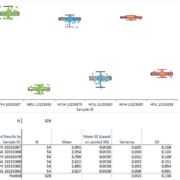
The analytical performance of the DxN VERIS System enabled the laboratory to consider the possibility of reducing the frequency of QC testing required to monitor routine patient analyses. In order to determine an appropriate QC frequency for viral load testing, the laboratory evaluated the performance characteristics of the analyser as well as the number of tests performed over a period time. Based on this assessment the laboratory concluded that performing three levels of QC (negative, low, high) two times per week would provide an acceptable level of system control while significantly reducing QC costs and hands-on time. This level of QC testing was appropriate based on the volume of tests performed by the laboratory. For labs that perform a higher volume of tests, QC may need to be performed more frequently in order to provide a sufficient level of QC relative to the number of tests performed. In case of QC out-of range, a procedure has been set-up in Rennes in order to re-test all samples analysed between the two QC measurement times. To validate the twice-weekly QC protocol, the lab evaluated the precision performance of each assay over 5 months. The precision observed over this time frame was less than 5.8% CV with standard deviation (SD) within 0.16 log IU/ml. No values were observed outside of the expected range. These data, summarized below, were determined by Rennes to be sufficient to support the twice-weekly QC protocol.
The Virology laboratory at Rennes University Hospital has been following the twice-weekly QC protocol since April 2016. This has resulted in a reduction in the cost per reportable result and simplified the QC process without impacting the quality of results produced by the lab.
Conclusion
Beckman Coulter’s DxN VERIS System provides a high level of assay performance, ease of use, and workflow efficiency. Effective quality control programs can be developed based on the unique testing requirements of each laboratory, resulting in a high level of system control while reducing hands-on time and QC cost.
* DxN VERIS products are CE-marked IVDs. DxN VERIS product line has not been submitted to U.S. FDA and is not available in the U.S. market. DxN VERIS Molecular Diagnostics System is also known as VERIS MDx Molecular Diagnostics System and VERIS MDx System.
The authors
J. Wyatt, P. Le Roux, V. Thibault1
Beckman Coulter Diagnostics | Brea, CA
1 Rennes University Hospital, France
2 CLSI. Statistical Quality Control for Quantitative Measurement Procedures:
Principles and Definitions; Approved
Guideline-Third Edition. CLSI document C24-A3. Wayne, PA: Clinical and
Laboratory Standards Institute.