Recent progress in laboratory diagnosis of hereditary spherocytosis
Hereditary spherocytosis is an inherited haemolytic anaemia due to fragile red cells. This article gives a brief overview of the pathophysiology of this red cell disorder, and presents the key points on the different screening tests.
by Dr May-Jean King
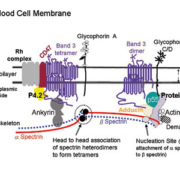
Membrane structure of human red blood cell and associated defects
The human red blood cell (RBC) is discoid or biconcave in shape. It deforms when navigating through blood vessels and capillaries. The integrity and elasticity of RBC are maintained and regulated by a series of interactions between two layers of proteins localised to the outer lipid bilayer and the cytoskeleton on the cytoplasmic side [Figure 1]. The resulting RBC membrane is a 3D structure composed of specific transmembrane proteins (the band 3 macro-complex, and the glycophoein C-protein 4.1R) and a 2D network of skeleton proteins spectrin, actin, protein 4.1R and other minor components [1]. A qualitative or quantitative abnormality in one of these membrane proteins will lead to fragile red cells and haemolytic anaemia. Hereditary spherocytosis is associated with defects in the vertical interaction of the band 3 macrocomplex (i.e., band 3, CD47, and Rh complex) with protein 4.2, and ankyrin to which β-spectrin binds directly [Figure 1]. Hereditary elliptocytosis has abnormalities in protein 4.1R or defective spectrin self-association [2]. A partial deficiency of protein 4.1R can affect its interaction with glycophorin C and P55 in a junctional complex, which is stabilised by a band 3-adducin-spectrin bridge [3]. The mutations located in the self-association site for spectrin αβ heterodimers can affect the formation of tetramers or higher oligomers that enable the extension of the spectrin-based cytoskeleton to cover the cytoplasmic side of the red cell membrane. HS and hereditary elliptocytosis are not single-gene diseases.
Hereditary spherocytosis
Hereditary spherocytosis (HS) is more prevalent among the Northern European populations (about 1 in 2000 to 5000 births) than in other ethnic groups. Where the cytoskeleton fails to attach to band 3 in the membrane via protein 4.2 and ankyrin, that area of membrane becomes detached and is pinched off from the intact RBC. This continuous loss of membrane lipid and integral membrane proteins reduces the RBC volume and transforms it into a spherocyte. Splenic sequestration of spherocytes reduces their lifespan in circulation to <120 days. Therefore a patient with HS presents a haemolytic anaemia with reticulocytosis, jaundice and possibly gallstones and/or splenomegaly [4]. The clinical phenotype of HS is heterogeneous, ranging from asymptomatic, mild, moderate to severe haemolysis requiring blood transfusion. HS is diagnosed in newborn or as late as in the fifth to seventh decade of life. A mild HS condition can be exacerbated by an infection (e.g., Parvovirus B19, CMV, Herpes 6, gastroenteritis), resulting in a severe haemolytic anaemia.
Laboratory testing for HS
Membranopathy is suspected when the cause of haemolytic anaemia remains unknown after the exclusion of enzymopathy, haemoglobinopathy and other extrinsic factors. Finding spherocytes in a blood smear indicates HS, but is not necessarily definitive. Exclusion of immune haemolytic anaemia (AIHA) is important because this condition also presents with spherocytosis [5]. Typical HS is expected to present almost all of the following features: evidence of a haemolytic process (e.g., raised bilirubin and LDH, low or no haptoglobin), low Hb, reduced mean cell volume, elevated mean cell haemoglobin concentration, and raised reticulocyte count [Figure 2]. The raised MCHC and increased % hyperdense RBCs are useful markers [6]. The diagnosis of dominant HS (75% of cases) is straightforward when family history of HS and the results for the red cell indices and blood chemistry are available. In the case of recessive HS, the proband may present a severe haemolytic anaemia with the blood smear showing anisocytosis and occasional cell fragments whereas the parents are apparently asymptomatic.
The majority of subjects with HS can be diagnosed by using a screening test without resorting to further investigation [Figure 2]. Two traditional screening tests for HS are still in use: the osmotic fragility test [7] and the acid glycerol lysis time test [8] [Table 1]. The cryohaemolysis test uses a change in temperature to effect red cell lysis [9]. The ektacytometer gives specific deformability profiles for a range of red cell disorders [Table 1]. However, this technique can give similar profiles for both HS and AIHA. SDS-polyacrylamide gel electrophoresis of erythrocyte membrane proteins is the confirmatory test because it detects all the membrane proteins known to be associated with HS [Figure 3, panel I]. Molecular analysis of membrane protein genes is usually performed by research laboratories. However, knowing the membrane protein defects and the associated protein gene mutation(s) does not influence the management of HS patients [12]. Unlike the aforementioned HS screening tests, the unusual feature of the EMA (eosin-5’-maleimide) Binding test [13] is the use of a flow cytometer, which analyses individual intact RBC in a sample. Confocal microscopy of EMA-labelled RBCs showed emission of both green and red fluorescence. RBCs of different sizes and shapes are labelled [Figure 3, panels II and III], [14]. The test is robust, only a low volume of patient specimen (5 µL packed RBC) and test reagent is required, and the test gives consistently reproducible results.
Conclusion
There is no screening test that has 100% sensitivity and 100% specificity for the diagnosis of HS. The adoption of the EMA Binding test is because it is easy to use and an abnormal result often indicates a membrane-associated red cell disorder. When this flow method is used in conjunction with the Osmotic Fragility test, differential diagnosis of HS and hereditary stomatocytosis can be made [described in 12].
References
1. Mohandas N & Gallagher PG. Red cell membrane: past, present, and future. Blood 2008; 112: 3939-3948.
2. Gallagher PG. Update on the clinical spectrum and genetics of red blood cell membrane disorders. Current Hematol Reports 2004; 3: 85-91.
3. Anong W et al. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton, and regulates membrane cohesion. Blood 2009; 114: 1904-1912.
4. Perrotta et al. Hereditary spherocytosis. Lancet 2008; 372:1411-1426.
5. Packman CH. The spherocytic haemolytic anaemias (historical review). Br J Haematol 2001;112: 888-899.
6. Cynober T et al. Red cell abnormalities in hereditary spherocytosis: relevance to diagnosis and understanding of the variable expression of clinical severity. J Lab Clin Med 1996;128:259-269.
7. Parpart AK et al. The osmotic resistance (fragility) of human red cells. J Clin Invest 1947; 26: 636-640.
8. Zanella A et al. Acidified glyceraol lysis test: a screening test for spherocytosis. Br J Haematol 1980; 45:481-486.
9. Streichman S & Gescheidt Y. Cryohemolysis for the detection of hereditary spherocytosis: correlation studies with osmotic fragility and authemolysis. A J Hematol 1998; 58:206-212.
10. Clark MR et al. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood 1983; 61: 899-910.
11. Johnson RM & Ravindranath Y. Osmotic scan ektacytometry in clinical diagnosis. J Ped Hematol Oncol 1996; 18: 122-129.
12. Bolton-Maggs et al. Guidelines for the diagnosis and management of hereditary spherocytosis – 2011 update. Br J Haematol 2011; doi:10.1111/j.1365-2141.2011.08921.x
13. King M-J et al. Rapid flow cytometric test for the diagnosis of membrane cytoskeleton-associated haemolytic anaemia. Br J of Haematol 2000; 111: 924-933.
14. King M-J et al. Using the eosin-5-maleimide binding test in the differential diagnosis of hereditary spherocytosis and hereditary pyropoikilocytosis. Cytometry Part B 2008; 74B: 244-250.
15. wKing M-J et al. Eosin-5-maleimide binding to band 3 and Rh-related proteins forms the basis of a screening test for hereditary spherocytosis. Br J Haematol 2004; 124:106-113.
The author
May-Jean King
Membrane Biochemistry
NHS Blood and Transplant
North Bristol Park
Filton
Bristol BS34 7QH
UK
e-mail: may-jean.king@nhsbt.nhs.uk