Serum B-cell maturation antigen: a novel marker for multiple myeloma
B-cell maturation antigen (BCMA) was originally identified as a cell surface receptor expressed on late-stage B cells, plasma cells, and B-cell malignancies including multiple myeloma (MM). We recently discovered that BCMA is shed into the blood of MM patients, and, therefore, serum BCMA may serve as a new prognostic marker to track disease status and response to treatment.
by Eric Sanchez, Suzie Vardanyan, Mingjie Li, Cathy Wang, Dr Haiming Chen, and Dr James R. Berenson
Background
B-cell maturation protein (BCMA, also referred to as TNFRSF17 or CD269) is a receptor shown to be expressed in B lymphocytes and at increasing levels as these cells mature [1]. BCMA binds the ligands BAFF (B-cell activating factor) and APRIL (a proliferation inducing ligand) [2, 3]. Membrane-bound expression of BCMA has been demonstrated in human CD138-expressing cells from multiple myeloma (MM) bone marrow (BM) and MM cell lines [4]. It has also been shown to be expressed on malignant cells from Hodgkin’s lymphoma and Waldenstrom’s macroglobulinemia (WM) patients [5, 6]. Although the response to treatment of MM patients is traditionally followed with measurement of their monoclonal immunoglobulin (Ig) levels, some patients do not produce this marker; and, moreover, in others, it is lost during the course of their disease.
Serum BCMA levels are elevated in MM patients
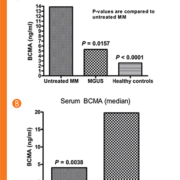
We measured serum BCMA by ELISA from patients with newly diagnosed MM, monoclonal gammopathy of undetermined significance (MGUS) and healthy control subjects. Using the International Staging System, 30, 12 and 7 patients with stages 1, 2 and 3, respectively, and one with unknown staging were analysed. We found that the serum levels of BCMA in MM patients (13.87 ng/ml) were elevated when compared to healthy controls (2.57 ng/ml; P <0.0001) and MGUS individuals (5.30 ng/ml; P = 0.0157; Fig.1A). We then determined that serum BCMA levels correlated with the MM patient’s response to therapy. Patients responding to their treatment regime had lower serum BCMA levels than patients with progressive disease (P = 0.0038; Fig. 1B). To confirm that the BCMA found in the blood of MM patients came from cells within the BM, BM aspirates were obtained from MM patients and BM mononuclear cells (MCs) were isolated and cultured for 48 hours. MM patients showed high levels of BCMA in culture medium whereas healthy subjects lacked significant amounts of BCMA. Moreover, serum and supernatant BCMA levels from MM patients were compared and showed a strong correlation (r = 0.82) between the levels in the serum and supernatants from cultured MM BMMCs (Fig. 2).
To exclude the possibility that the BCMA detected in MM patient serum and from cultured MM BMMCs may have been derived from non-malignant cells, we evaluated human BCMA levels in our human MM xenografts growing in severe combined immunodeficient (SCID) mice. Animals were implanted with the human MM tumour LAGκ-2 and analysed for human serum BCMA levels. SCID mice dosed with bortezomib (0.5 mg/kg, twice weekly) had a reduction in tumour volume compared to untreated mice (P = 0.0067; Fig. 3A), and human serum BCMA levels from these bortezomib-treated animals were also markedly lower compared to untreated mice (P = 0.0006; Fig. 3B). Similar results were obtained when using our other MM xenograft models (LAGλ-1, LAGκ-1A). Human BCMA was not detected in the serum of non-tumour-bearing mice (data not shown). A rise in serum BCMA levels from the possible release of membrane-bound protein from dead MM cells was not observed in mice following drug treatment. Thus, it can be concluded that the serum levels of BCMA in the mice are derived from live MM cells.
Soluble BCMA has been shown to inhibit normal B-cell development through interference with the binding of its ligands (BAFF and/or APRIL) to membrane-bound BCMA on normal B cells. One group demonstrated that administration of BCMA–Ig fusion protein to normal mice, which inhibits the binding of BAFF to B cells, resulted in a dramatic reduction in B-cell numbers in the blood and peripheral lymphoid organs [2]. Splenic B-cell reductions were shown to occur in an in vitro mouse splenocyte proliferation assay following in vivo administration of BCMA–Fc fusion protein to normal mice [7]. Other investigators have shown that injecting soluble BCMA–Fc fusion protein, which binds BAFF and APRIL both as free and membrane-bound ligands, into nude mice bearing human colon or lung carcinoma cell lines resulted in inhibition of tumour growth [3]. The authors suggested that BCMA bound its ligand APRIL, and prevented the known stimulatory effect of APRIL on these cell lines. Additionally, investigators have shown in vitro the existence of BCMA–BAFF complexes [3, 7, 8]. In the context of cancer growth, one group demonstrated that BCMA injected into mice reduced the growth of tumour cells from human colon or lung carcinoma cell lines in vivo by binding its ligand APRIL [3]. Thus, we are currently conducting studies to determine if such complexes exist in vivo and may block the immune function of myeloma patients.
Conclusions and future directions
Measurement of MM tumour mass is indirect, given the location of this BM based malignancy. Thus, assessment of tumour burden in response to therapy is difficult but essential to effectively monitor patients with MM. Traditionally, changes in Ig levels have been used to follow disease progression and response to treatment. In fact, for three decades Ig was a prognostic factor used in the Durie-Salmon staging system [9], which, until recently, was the most widely used staging system [10]. However, assessment of this protein does not always accurately reflect changes in MM tumour burden [11–13]. Additionally, small subsets of MM patients do not produce this marker. Thus, additional markers are needed to assess response to therapy in these non-secretory patients and in MM patients as a whole.
We have now shown that BCMA is present in the serum of MM patients; and, moreover, its levels correlate with the patient’s response to therapy [14]. We have also previously shown that supernatant from cultured BMMCs from MM patients with active disease contain much higher levels of this protein in their culture medium than in healthy subjects and those with MGUS or indolent MM [14]. SCID mice bearing human MM xenografts also showed high levels of BCMA in their sera, and these levels decreased in response to anti-MM therapy. We believe that that serum BCMA will eventually be used in the clinic as a diagnostic and prognostic marker for MM patients.
References
1. Laabi Y, Gras MP, Brouet JC, et al. The BCMA gene, preferentially expressed during lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Res. 1994; 22(7): 1147–1154.
2. Thompson JS, Schneider P, Kalled SL, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000; 192(1): 129–135.
3. Rennert P, Schneider P, Cachero TG, et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis family member APRIL, inhibits tumor cell growth. J Exp Med. 2000; 192(11): 1677–1684.
4. Novak AJ, Darce JR, Arendt BK, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood 2004; 103(2): 689–694.
5. Chiu A, Xu W, He B, et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood 2007; 109(2): 729–739.
6. Elsawa SF, Novak AJ, Grote DM, et al. B-lymphocyte stimulator (BLyS) stimulates immunoglobulin production and malignant B-cell growth in Waldenstrom macroglobulinemia. Blood 2006; 107(7): 2882–2888.
7. Pelletier M, Thompson JS, Qian F, et al. Comparisons of soluble decoy IgG fusion proteins of BAFF-R and BCMA as Antagonist for BAFF. J Biol Chem. 2003; 278(35): 33127–33133.
8. Shu HB, Johnson H. B cell maturation protein is a receptor for the tumor necrosis factor family member TALL-1. Proc Natl Acad Sci U S A 2000; 97(16): 9156–9161.
9. Durie BG, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer 1975; 36(3): 842–854.
10. Larson RS, Sukpanichnant S, Greer JP, et al. The spectrum of multiple myeloma: diagnostic and biological implications. Hum Pathol. 1997; 28(12): 1336–1347.
11. Sullivan PW, Salmon SE. Kinetics of tumor growth and regression in IgG multiple myeloma. J Clin Invest. 1972; 51(7): 1697–1708.
12. Kawano M, Huang N, Harada H, et al. Identification of immature and mature cells in the bone marrow of human myelomas. Blood 1993; 82(2): 564–570.
13. Zaanen HCT, Lokhorst HM, Aarden LA, et al. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. Br J Haematol. 1998; 102(3): 783–790.
14. Sanchez E, Li M, Kitto A, et al. Serum B-cell maturation is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012; 158(6): 727–738.
The authors
Eric Sanchez BA; Suzie Vardanyan BS; Mingjie Li BS; Cathy Wang BS; Haiming Chen MD, PhD; and James R. Berenson* MD
Institute for Myeloma & Bone Cancer Research, West Hollywood, CA, USA
*Corresponding author
E-mail: Jberenson@imbcr.org