Sleeping sickness elimination: are we dreaming?
Recent sleeping sickness epidemics killed over 400,000 people in less than 20 sub-Saharan African countries. Serological screening of populations at risk and treatment of confirmed patients have drastically reduced the annually reported cases. Elimination seems feasible but only with new control tools and strategies adapted to the new epidemiological situation.
by Dr Philippe Büscher, Quentin Gilleman and Dr Pascal Mertens
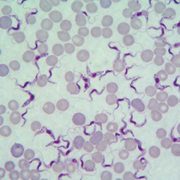
Sleeping sickness, also called human African trypanosomiasis (HAT), is caused by two subspecies of the protozoan parasite Trypanosoma brucei (T.b.). The disease is transmitted by blood sucking tsetse flies that only occur in sub-Saharan Africa. T.b. gambiense causes a rather chronic disease and is found in West and Central Africa. T.b. rhodesiense causes a more fulminant form of the disease in Eastern Africa. Other Trypanosoma species cause diseases in animals, including cattle and small ruminants [Figure 1].
Infection and pathology
After inoculation with the saliva of an infective tsetse fly, the parasites invade lymph, blood and all peripheral organs where they multiply and survive the immune response of the host by a biological mechanism called antigenic variation. Eventually, the parasites invade the brain causing intrathecal inflammation associated with neurological disorders such as altered sleep-wake rhythm, behavioural changes, motor disabilities etc.
Except for some very rare cases, the disease is always lethal and even after successful treatment, many patients, especially children, never recover completely and remain disabled for the rest of their life. Sleeping sickness is a rural disease affecting poor populations living in the forests and wooded savannah where tsetse flies breed. Today, T.b. rhodesiense is mainly found in wild animals in game parks and natural reserves where it is often transmitted to rangers and visiting tourists.
Epidemiological background
At the turn of the 20th century, both gambiense and rhodesiense sleeping sickness caused devastating epidemics killing about one million people within two decades. By sustained implementation of vector control (including habitat destruction and insecticide spraying), culling of wild animals, and systematic screening of the population and treatment of patients by specialized teams, the colonial governments gained control over the epidemics and reduced the annual number of cases to less than 5000 cases around 1960. However, around 1990, a new epidemic of gambiense HAT was rampant in many countries with several tens of thousands of annually reported patients [1]. Countries most affected were typically poor and socio-politically unstable such as Angola, Central African Republic, D.R. of the Congo, Rep. of Congo, Sudan and Uganda to name a few.
Current situation
Today, about 20 years later, the number of reported cases has fallen again to about 7000 in 2012 of which 85% were diagnosed and treated in one single country, the D.R. of the Congo [2]. This achievement was made possible by a combination of different factors among which the availability of performing diagnostic tests and effective treatment, the recognition of sleeping sickness as Neglected Tropical Disease (NTD), thus attracting attention by donor agencies, humanitarian organizations and the private sector, and the combined effort of the World Health Organization, national HAT control programmes, bilateral cooperations and Non Governmental Organizations to organize large scale active case finding in the affected regions.
Active case finding is typically done by mobile teams that consist of up to seven persons trained in diagnosis and treatment of HAT. They go out in the field for several weeks, carrying all necessary equipment, diagnostics and drugs to screen the population at risk with a serological antibody detection test, to examine seropositive suspects by microscopy and to treat parasitologically confirmed patients in their villages or to refer them to the nearest specialized treatment centre. Since more than 20 years now, the recommended screening test is the Card Agglutination Test for Trypanosomiasis (CATT), a rapid test that detects gambiense specific antibodies [3].
Neglected Tropical Disease (NTD)
The recent success in HAT control has led to the inclusion of gambiense HAT in the WHO’s list of NTD’s that could be eliminated as a public health problem in Africa by 2020 with zero transmission in 2030 [2]. However, with the currently available tools for HAT control, elimination may remain an elusive target. Indeed, eradication of the tsetse flies, although proven to be feasible in some isolated foci with only one species transmitting trypanosomes, probably will never be achieved in endemic countries with dense forests and with large protected zones. As a consequence, tsetse flies will continue to transmit the disease, not only from man to man but also from the domestic and wild animal reservoir to man.
Diagnosis and treatment of infection
Today, treatment of sleeping sickness patients relies on toxic drugs and most often requires several weeks of hospitalization. Therefore, treatment is
administered only to patients in which the parasites have been detected in the blood, lymph or cerebrospinal fluid. Given that even the most sensitive parasite detection tests remain negative in 10% to 20% of actually infected patients, untreated patients may continue to act as a parasite reservoir, sometimes for years before they are treated or die. With the venue of molecular diagnostics, it was believed that such tests would sooner or later replace microscopic parasite detection. However, HAT patients have to be diagnosed in rural environments that are not compatible with today’s DNA- or RNA-based diagnostics and molecular test do not perform better than parasitology. Therefore, it is questionable if the individual patient will ever benefit from molecular diagnostics for sleeping sickness [4].
New control tools
Should we then despair about sleeping sickness elimination? Not at all, at least not for gambiense HAT. History shows that in countries that are socio-politically stable, where the rural population has access to functional primary healthcare facilities and where changing land use has suppressed the tsetse fly population, sleeping sickness has disappeared as is the case in Benin, Burkina Faso, Ghana and Togo [5]. For countries where these conditions cannot be met in the near future, newly developed HAT control tools may play a major role in disease elimination. For example, GIS technology allows to combine the GPS coordinates of all villages where HAT patients are reported with demographic and environmental data, and to precisely map the populations at risk [6].
New rapid test
Also, a new rapid diagnostic test for gambiense HAT serodiagnosis has been developed (HAT Sero-K-SeT, Coris BioConcept, Belgium). The HAT Sero-K-SeT is individually packed, thermostable, equipment-free , robust and has shown excellent diagnostic performance in a phase I evaluation [7]. Its target product profile, and especially its very high specificity, makes it fully compatible for use in foci with very low prevalence and in fixed health centres with minimal infrastructure [Figure 2]. In addition, strategies involving newly developed small size (0,25 x 0,25 m) insecticide-treated targets to kill the riverine tsetse fly are more cost effective than former models [8].
New drugs in the pipeline
Finally, the search for new drugs has identified a new class of compounds of which one, the SCYX-7158 has been selected for the development of a safe, one-dose oral treatment of both stages of sleeping sickness [9]. Once such a drug becomes available, parasite detection and stage determination that can only be accurately performed by expert medical staff, may become dispensable and decision to treat might be taken on the serodiagnostic evidence of infection.
Conclusion
Elimination of at least one form of sleeping sickness seems possible but only with the long-term commitment of donor agencies and ministries of health in endemic countries and with the cost efficient deployment of the newly developed control tools in rationally designed elimination strategies adapted to the local epidemiological situation.
References
1. World Health Organization. Control and surveillance of African trypanosomiasis. WHO Technical Report Series 1998; 881: 1-113.
2. World Health Organization. Report of a WHO meeting on elimination of African trypanosomiasis (Trypanosoma brucei gambiense), 3-5 December 2012, Geneva, Switzerland. WHO/HTM/NTD/IDM 2013.4 http://apps.who.int/iris/bitstream/10665/79689/1/WHO_HTM_NTD_IDM_2013.4_eng.pdf (accessed 27 May 2013)
3. Chappuis F, Loutan L, Simarro P, Lejon V and Büscher P. Options for the field diagnosis of human African trypanosomiasis. Clinical Microbiology Reviews 2005; 18: 133-146.
4. Deborggraeve S and Büscher P. Molecular diagnostics for sleeping sickness: where’s the benefit for the patient? The Lancet Infectious Diseases 2010; 10: 433-439.
5. Simarro PP, Diarra A, Ruiz Postigo JA, Franco JR, and Jannin JG. The human african trypanosomiasis control and surveillance programme of the world health organization 2000-2009: the way forward. PLoS Neglected Tropical Diseases 2011; 5: e1007.
6. Simarro PP, Cecchi G, Franco JR et al. Estimating and mapping the population at risk of sleeping sickness. PLoS Neglected Tropical Diseases 2012; 6: e1859.
7. Büscher P, Gilleman Q and Lejon V. Novel rapid diagnostic tests for sleeping sickness. New England Journal of Medicine 2013; 368: 1069-1070.
8. Esterhuizen J, Rayaisse JB, Tirados I et al. Improving the cost-effectiveness of visual devices for the control of riverine tsetse flies, the major vectors of human African trypanosomiasis 3. PLoS Neglected Tropical Diseases 2011; 5: e1257.
9. Jacobs RT, Nare B, Wring SA et al. SCYX-7158, an Orally-Active Benzoxaborole for the Treatment of Stage 2 Human African Trypanosomiasis. PLoS Neglected Tropical Diseases 2011; 5: e1151.
The authors
Philippe Büscher1* PhD, Quentin Gilleman2 MSc, and Pascal Mertens2 PhD
1 Institute of Tropical Medicine, Department of Biomedical Sciences, Nationalestraat 155, B-2000 Antwerp, Belgium
2 Coris BioConcept, Crealys Park, Rue Jean Sonet 4a, B-5032 Gembloux, Belgium
*Corresponding author
E-mail: pbuscher@itg.be
Tel. +32 3247 6371