The Ebola Spatial Care Path : point – of – care lessons learned for stopping outbreaks
Ebola profoundly elevated the impact of point-of-care testing, now recognized worldwide as essential to detect the disease, reduce risk, monitor patients in isolation, achieve recovery, and importantly, contain outbreaks. The goal is to become resilient – a new and possibly more contagious threat might appear. We must stop it where it starts!
by Prof. G. J. Kost, W. Ferguson, A.-T. Truong, D. Prom, J. Hoe, A. Banpavichit and S. Kongpila
Introduction – the essential role of point-of-care testing
Point-of-care testing (POCT) is propelling the convergence, integration and sustainability of global diagnostics. We should not be caught off guard at points of need! Using fever to screen patients for Ebola virus disease (‘Ebola’) occurs too far downstream in the clinical course, casts an excessively wide net confounded by other febrile illnesses, defeats rapid epidemiological control of outbreaks and inhibits evidence-based karma essential for compatible point of care culture. In fact, poor focus misleads the public, who, once cognizant of the essential role, importance and comprehensiveness of rapid POC diagnosis, will be receptive to containment and disposed to enter treatment centres, if they are more certain they have Ebola.
The Ebola ‘newdemic’ (an unexpected and disruptive problem that affects the health of large numbers of individuals in a crowded world) moved POCT from parochial fiduciaries often stalled by analysis paralysis to action-oriented value generators, that is, inventors and innovators leading the way with next-generation technologies and high stakes strategies, as summarized in this article, which are beneficial for reducing risk and enhancing resilience. It inspired the Ebola Spatial Care Path™ (SCP) and a useful Diagnostic Centre (DC) design equipped with POCT, presented here as well [1].
Rapid evolution of diagnostic tests for Ebola virus disease
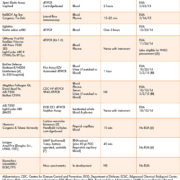
Table 1 chronicles the pioneering ongoing efforts of industry, academia and government to produce workable immunoassays and molecular diagnostics for the detection of Ebola. In fact, this research development will spill over to energize POC diagnostics for highly infectious diseases in general. Novel research also is exploring digital detection of Ebola virus and viral load, which is higher in fatal cases and may be related to the development of virus-induced shock. Aside from the logistic challenges of getting assays ready in time, new assays, which might be implemented on instruments like the GenePOC, must be proven to work in clinical studies. As far back as 2006, investigators reviewed laboratory diagnostics for Ebola. Now, nearly a decade later, the FDA is accelerating the ongoing development, validation and approval of new diagnostic tests by issuing emergency use authorizations (EUAs) more or less continuously since autumn 2014 (Table 1).
Ebola-specific challenges for molecular diagnostics include: (a) reduction in initial false negatives (FN), FN = FN(t), as a function of time, to ramp up sensitivity, {TP/[TP + FN(t)]} (where TP=true positive), to ultrahigh levels in infected patients during the first 72 hours when symptoms may be mild or absent, in order to avoid shunting false negative cases to community hospitals ill prepared to receive high-risk patients; (b) automation of totally self-contained and sealable specimen cassettes and cartridges to eliminate need for expensive high-level biosafety cabinets; (c) proof of effectiveness in controlling internal contamination in portable instruments, thereby sustaining high specificity [TN/(TN + FP)] (where TN=true negative) and minimizing false positives (FP), which place people at risk when near infected patients; and as more sophisticated but compact technologies become available in the future, (d) determination of quantitative viral genome titers, which will be useful for early detection of exposure in smaller volumes of specimen and also for de-escalating the level of care and quarantine as the patient improves.
When performed properly with biohazard precautions in the near-patient testing area of a DC, results will be available much more quickly than sending specimens to a public health laboratory or to the Centers for Disease Control and Prevention (CDC). The gain in time can be substantial, just 1 hour or less needed to obtain an answer (see Table 1), which facilitates rapid screening, focused triage, and effective workflow. Self-contained cartridge/cassette-based rapid molecular tests are available on small portable platforms that test for infectious diseases. Development of POC molecular diagnostics for high risk infectious diseases forecasts the feasibility of introducing Ebola assays on light-weight platforms, such as the Alere I (see http://www.alere.com/us/en.html), and the tiny light-weight Roche Diagnostics cobas Liat (see https://usdiagnostics.roche.com/en/instrument/cobas-liat.html); both of these nucleic acid testing devices are Clinical laboratory Improvement Amendments (CLIA)-waived, user-friendly and, therefore, good candidates for point-of-need testing.
If tests satisfy certain conditions, they can be ‘waived’. In other words, the tests are cleared by the US Food and Drug Administration (FDA) to be performed in clinics and possibly even at home. Testing is simple to carry out and the instruments are operator-friendly, which make chances of an inaccuracy less likely. Such tests are referred to as a CLIA-waived. We will see facilitated-access, self-testing (FAST) POC solutions emerge as industry moves forward in the chronological evolution of Ebola EUAs in Table 1, some of which will be appearing commercially as inexpensive, portable, safe, and appropriate for detection of virus in the early stages of clinical illness. True, we are behind on the timeline. However, the good news is that everyone recognizes the need, the problem has been defined, POCT is part of the solution, and the feasibility of immediate testing at points is proven, as summarized in Table 2.
The Ebola Spatial Care Path
We define a Spatial Care Path (SCP) as the most efficient route taken by the patient when receiving definitive care in a small-world network (SWN). SCP principles include: (a) start diagnosis immediately wherever the patient is located; (b) implement POC technologies according to needs in the home, ambulance, primary care, SWN hubs, and at the bedside in critical care; (c) thereby achieve timely evidence-based decision making based on POC test results as the patient progresses through the SWN of healthcare; (d) coordinate access to the most critical elements and scarce specialists of the SWN to achieve a continuum of care; and (e) optimize the use of medical resources for the problem at hand, especially when the SWN becomes compromised or patients are selectively quarantined.
Spatial in this definition refers to shrewd positioning of POCT, elimination of unnecessary process steps, use of geographic information systems (GISs) to identify effective and efficient routes from population clusters to the nearest medical care, and in the case of Ebola, consolidation of SWN dispersion into one or more community alternative care facilities (ACFs) and DCs in which the useable space and workflow are optimized. Figure 1 illustrates the Ebola SCP with ACF and embedded POCT (on the left) integratively connected to a current expedient solution (on the right) of an individual hospital isolation area with a limited number of beds. A strategic Ebola SCP will deploy the best available molecular diagnostic testing at the point of initial patient contact and eliminate time-consuming steps in the sequence of care, such as transporting high risk Ebola patients from one community to another or sending hazardous samples to reference laboratories in heavily populated cities. Designing SCPs will facilitate prevention, intervention, and resilience in the event of wider presence of Ebola and simultaneously, will fulfill community recommendations of the CDC. We propose that each regional SWN analyse and ready its own SCP with POCT.
The Diagnostic Centre and interpretation of test results
Figure 2 shows the DC designed for Ebola care in Southeast Asia. POCT within the biosafety cabinet (top left) comprises: (a) the Spotchem EZ (Arkray, http://www.arkrayusa.com/) for determination of glucose, total protein, albumin, ALT, AST, alkaline phosphatase, cholesterol, triglycerides, HDL, urea nitrogen, creatinine, calcium, and total bilirubin, or combinations thereof (this instrument has been used for support of patients with viral hemorrhagic fever in Ghana); (b) the Opti CCA-TS2 whole blood analyser (http://www.optimedical.com/products-services/opti-CCA-TS2.html) for measurements of pH, pCO2, pO2, total hemoglobin, oxygen saturation, Na+, K+, Ca++ (ionized or free calcium), Cl−, glucose, urea nitrogen, and lactate, but only eight of these analytes at one time using a directly loading syringe cartridge that minimizes contamination; (c) a hematology instrument (optional), such as the QBC Star (http://www.druckerdiagnostics.com/hematology/qbc-star/qbc-star-centrifugal-hematology-analyzer.html), a dry reagent analyser that produces a nine-component complete blood count [hematocrit, hemoglobin, MCHC (mean corpuscular hemoglobin concentration), platelet count, white blood cell count, granulocyte count and percentage, and lymphocyte/monocyte count and percentage] from a specialized sample tube with stains and float separator inside, or the HemoCue CBC-DIFF (http://www.hemocue.com/en/products/white-blood-cell-count/wbc-diff); and within the isolation confines, (d) a vital signs monitor (e.g. VTrust TD-2300).
Premonitory POC test results, such as initial leukopenia, suppressed lymphocyte count on the differential, increased percentage of granulocytes and thrombocytopenia help confirm the diagnosis of Ebola. Later, patients have increased white blood cells (WBC), immature granulocytes and atypical lymphocytes. West Africa should be replete with POCT and DCs, but is not, thereby handicapping expeditious detection of premonitory signs and evidence-based critical care support in treatment centers. Striking electrolyte changes need monitoring to support repletion. Unfortunately, there is no small FDA-cleared handheld device for monitoring of coagulation (except PT/INR when adjusting warfarin anticoagulant, where PT is prothrombin time and INR is international normalized ratio). Filoviral hemorrhagic fever is accompanied by prolonged PT, activated PTT and bleeding time, potentially progressing to DIC with elevated D-dimer. D-dimer is available on the handheld cobas h232 (Roche Diagnostics, http://www.cobas.com/home/product/point-of-care-testing/cobas-h-232.html) available outside the U.S. As demonstrated by the two recent U.S. Ebola patients, platelets are consumed rapidly early in the course of the infection, and should be trend mapped to see recovery, possibly along with assessment of platelet function. Note that fatally infected patients fail to develop an antibody response. Thus, the detection of virus-specific IgM and IgG is a good prognostic sign. In critically ill Ebola patients, plasma loss and bleeding affect hemoglobin and the hematocrit, both of which should be monitored at the point of care.
Conclusions
POCT is facilitating global health. Now, global health problems are elevating POCT to new levels of importance for accelerating diagnosis and evidence-based decision making during disease outbreaks. Authorities concur that rapid diagnosis has potential to stop disease spread. New technologies offer minimally significant risks for personnel and can be used in conjunction with risk prediction scores for patients. With embedded POCT, strategic SCPs planned by communities fulfill CDC recommendations. POC devices should consolidate multiplex test clusters supporting Ebola patients in isolation. The ultimate future solution is FAST POC. DCs in ACFs and transportable formats also will optimize Ebola SCPs. In short, POCT can help stop outbreaks.
Acknowledgements and disclaimer
Spatial Care Path™ is a trademark by William Ferguson and Gerald Kost, Knowledge Optimization®, Davis, CA. Figures and tables were provided courtesy and permission of Knowledge Optimization®, Davis, California, and Visual Logistics, a division of Knowledge Optimization®. Figure 2 was created by Lab Leader Company, Ltd., Bangkok, Thailand. Devices must comply with jurisdictional regulations in specific countries, operator use limitations based on patient conditions, federal and state legal statutes, and hospital accreditation requirements. Not all POC devices presented in this paper are cleared by the FDA for use in the U.S.A. FDA emergency use authorization is limited in scope and term. Please check with manufacturers for the current status of Ebola diagnostics and POC tests within the relevant domain of use.
References and notes
1. Kost GJ, Ferguson WJ, Hoe J, Truong A-T, Banpavichit A, Kongpila S. The Ebola Spatial Care Path™: accelerating point-of-care diagnosis, decision making, and community resilience in outbreaks. American Journal of Disaster Medicine 2015 [accepted for publication].
2. The FDA Emergency Use Authorization (EUA) status can be found at: http://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/MCMLegalRegulatoryandPolicyFramework/ucm182568.htm#current.
3. See WHO Emergency Quality Assessment Mechanism for EVD IVDs Public Report. Product: RealStar® Filovirus Screen RT-PCR Kit 1.0 Number: EA 0002-002-00. http://www.who.int/diagnostics_laboratory/procurement/141125_evd_public_report_altona_v1.pdf?ua=1.
4. FierceMedicalDevices. One-hour Ebola test receives FDA emergency use authorization. http://www.fiercemedicaldevices.com/story/one-hour-ebola-test-biom-rieux-receives-fda-emergency-use-authorization/2014-10-27.
5. Jones A, Boisen M, Radkey R, Bidner R, Goba A, Pitts K. Development of a multiplex point of care diagnostic for differentiation of Lassa fever, Dengue fever and Ebola hemorrhagic fever. American Association for Clinical Chemistry Poster. http://www.nano.com/downloads/Ebola%20testing_PCR%20vs%20Immunoassay.pdf.
6. Instrumentation and corporate/academic relationships may have changed. See ‘Letters of Authorization’ on the FDA EUA webpage for details. Contact company and investigator sources for updates.
7. Benzine J, Manna D, Mire C, Geisbert T, Bergeron E, Mead D, Chander Y. Rapid point of care molecular diagnostic test for Ebola virus. Poster at ASM-Biodefense 2015. http://www.douglasscientific.com/NewsEvents/News/2014-10-21%20Lucigen%20to%20Seek%20FDA%20Emergency%20Use%20Approval%20for%20Isothermal%20Point-of-Care%20Ebola%20Test.pdf
8. See Piccolo xpress for test clusters. http://www.piccoloxpress.com/products/panels/menu/.
9. See Siemens website for details. clinitekhttp://www.healthcare.siemens.com/point-of-care/urinalysis/clinitek-status-analyzer/technical-specifications.
10. FDA-cleared for warfarin monitoring only.
11. See Sysmex website for list of variables and parameters. https://www.sysmex.com/us/en/Brochures/Brochure_pocH-100i_MKT-10-1025.pdf for list of variables and parameters.
12. Ebola assay FDA-cleared for emergency use only.
13. Beckman-Coulter, La Brea, California, manufactures the DxI800 and DXC800i.
14. Walker NF, Brown CS, Youkee D, Baker P, Williams N, Kalawa A, et al. Evaluation of a point-of-care blood test for identification of Ebola virus disease at Ebola holding units, Western Area, Sierra Leone, January to February 2015. Euro Surveillance 2015; 20(12): pii=21073.
15. Owen WE, Caron JE, Genzen JR. Clin Chim Acta 2015; 446: 119-127.
16. Nicholson-Roberts T, Fletcher T, Rees P, Dickson S, Hinsley D, Bailey M, et al. Ebola virus disease managed with blood product replacement and point of care tests in Sierra Leon. QJM 2015; pii: hcv092 [advance access publication]. http://qjmed.oxfordjournals.org/content/qjmed/early/2015/05/07/qjmed.hcv092.full.pdf.
The authors
Gerald J. Kost* MD, PhD, MS, FACB (emeritus); William Ferguson BS, MS; Anh-Thu Truong; Daisy Prom; Jackie Hoe; Arirat Banpavichit MS, MBA; Surin Kongpila MS
Point-of-Care Center for Teaching and Research (POCT•CTR), School of Medicine, University of California, Davis, CA, USA
*Corresponding author
E-mail: gjkost@ucdavis.edu