The HDL particle: frontiers for new discovery in cardioprotection
Recent findings indicate that aspects of high-density lipoprotein (HDL) not captured by traditionally measured HDL–cholesterol levels (HDL‑C) are likely to be cardioprotective. This review will highlight some of these studies and suggest new directions to identify the specific molecules that are responsible for the cardioprotective nature of HDL.
by Daniel S. Kim, Dr Patrick M. Hutchins and Prof. Gail P. Jarvik
Raising HDL-C does not confer cardioprotection
There is a well-established inverse association between high-density lipoprotein–cholesterol (HDL-C) levels and cardiovascular disease (CVD) in epidemiological and clinical studies [1, 2]. This robust relationship suggested that HDL-C was in the causal pathway of atheroprotection. Indeed a large number of studies have demonstrated that HDL possesses various anti-atherogenic properties, primarily the ability to accept cholesterol from macrophages in a process termed reverse cholesterol transport [3, 4].
In contrast, several high-profile studies have demonstrated that increasing levels of HDL-C does not have a significant cardioprotective effect. In a large and well-conducted clinical trial of the cholesterol ester transport protein (CETP) inhibitor, torcetrapib, there was no reduction in the incidence of CVD-related events despite significantly higher HDL-C levels [5]. A follow-up study using a different CETP inhibitor, dalcetrapib, also showed increased HDL-C levels yet there was no significant difference in CVD event rate between the treatment and placebo groups [6]. In a third randomized clinical trial that used niacin to increase HDL-C levels, there was again no reduction in cardiovascular events [7]. Finally, a large-scale Mendelian randomization study of approximately 20 000 myocardial infarction (MI) cases and 100 000 controls, showed that a genetic polymorphism which associated with approximately 10% higher HDL-C levels was not associated with decreased incidence of MI [8], again suggesting that the relationship between HDL-C and the prevention of cardiac events is not causal.
HDL particle concentration is a superior predictor of CVD
As the elevation of HDL-C was not beneficial in these studies, some have speculated that HDL itself is not cardioprotective. An alternative explanation for these negative data is that the cholesterol content of HDL – a surrogate measure of HDL – does not best reflect the anti-atherogenic properties of HDL. To resolve these issues it is critical to identify new HDL metrics that reliably reflect its cardioprotective functions.
One promising approach for assessing the role of HDL in CVD is to evaluate the individual HDL particles. HDL is a heterogeneous mixture of lipoprotein particles composed of discrete subspecies that have unique structural compositions and biological functions. As different HDL particles carry vastly different amounts of cholesterol – ranging over an order-of-magnitude [9, 10] – measuring the total HDL-C does not provide information regarding the distribution of HDL subpopulations or the number of total HDL particles.
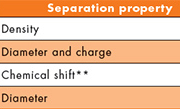
HDL can be fractionated based on a number of physicochemical properties, most commonly size or density. Several techniques, both qualitative and quantitative, have been developed for HDL subspecies analysis. The various HDL subspecies reported by these techniques and their associated nomenclature are briefly summarized in Table 1 [see also ref. 11]. Furthermore, HDL subspecies determined by ultracentrifugation and calibrated ion mobility analysis (both are discussed in detail later) are shown in Figure 1. Many studies have demonstrated the potential clinical utility of HDL subspecies analysis, which can be achieved by techniques such as 2D gradient gel electrophoresis [12] and nuclear magnetic resonance (NMR) [13]. For example, one study (using 2D gradient gel electrophoresis) showed that very-large, cholesterol-rich α-1 HDLs were better predictors than HDL-C levels of reduced coronary heart disease (CHD) in a subset of males from the Framingham Offspring Study [14]. Another high-profile study, using NMR to assess HDL subspecies in over 2200 participants in the EPIC-Norfolk cohort, showed that higher HDL particle (HDL-P) concentrations were a predictor of reduced CHD, independent of classic CHD risk factors [15]. In more recent work from the Multi-Ethnic Study of Atherosclerosis, total HDL-P (measured by NMR) and HDL-C were evaluated at baseline for 5598 participants, who were then followed prospectively for incident CHD (n=227 events) [16]. Although both HDL-P and HDL-C were highly correlated with each other, in multivariate regression models total HDL-P concentration was the superior predictor of reduced incident CHD when compared to HDL-C. This finding indicates that although HDL-C captures a large portion of HDL-P variation, HDL-P is the better predictor of CHD.
These studies support the notion that measuring individual HDL particle subspecies provides clinically useful information beyond traditionally measured HDL-C. However, both α-1 HDLs (which are cholesterol-rich) and HDL-P measured by NMR (which relies on lipid to generate signal) are highly correlated with HDL-C. Therefore, it is possible that these observations reflect a similar inverse association observed between HDL-C and cardiovascular disease. Importantly, two recent studies (discussed below) indicate that low levels of relatively cholesterol-poor, smaller HDLs also associate with cerebrovascular disease, again suggesting that subspecies of HDL not adequately captured by measuring HDL-C may also play important roles in the pathogenesis of atherosclerotic disease.
Shifting focus: HDL-3 and medium-HDL particles
We investigated the association of the subspecies HDL-2 and HDL-3 (Table 1; Fig. 1) with carotid artery disease (CAAD) [17]. Here, HDL was sub-fractionated by ultracentrifugation and the subspecies were quantified by their cholesterol content. In a case-control cohort of 1,725 participants [part of the Carotid Lesion Epidemiology And Risk (CLEAR) cohort], stepwise linear regression was used to determine whether total HDL-C, HDL-2 cholesterol (HDL-2C), HDL-3 cholesterol (HDL-3C), or apolipoprotein A-I (apoA-I) levels were the best predictor of CAAD. In this study, the smaller HDL-3C fraction was found to be the best predictor of reduced CAAD risk. Moreover, adding HDL-3C to the model improved prediction even when HDL-C levels were also considered, demonstrating added utility of the HDL-3C measure versus HDL-C.
In a separate study using calibrated ion mobility analysis, the particle concentrations of three HDL subspecies (Table 1; Fig. 1) were measured in a subset of the same CLEAR cohort [18]. Participants with severe carotid stenosis (n=40; >80% stenosis by ultrasound in either or both internal carotid artery) had significantly lower plasma concentrations of medium-HDL particles compared with control participants (n=40; <15% stenosis by ultrasound in both carotid arteries). In this population HDL-P was a superior predictor of CAAD compared to HDL-C and this relationship was significant after controlling for HDL-C. The case-control difference in total HDL-P was driven by dramatic changes in medium-HDL particles, the next best predictor of CAAD. This medium-HDL particle inverse association also remained significant after controlling for HDL-C. Considering HDL-3 is composed of small- and medium-HDL particles (Fig. 1) and medium-HDL contributes the majority of HDL-3 cholesterol content, these results are in excellent agreement with the previous study of the CLEAR cohort. Both results support the hypothesis that relatively cholesterol-poor, smaller HDL subspecies, which are under-represented by total HDL-C, are potentially important protective factors for CVD.
Summary and future directions
Considering that increased levels of cholesterol-poor HDL subspecies – reflected by measures of HDL-3, medium size particles, and increased HDL-P – can represent superior predictors of CVD phenotypes, it is possible that pharmacologic attempts to raise HDL-C fail to affect CVD event rates because specifically elevating the cholesterol content of HDL is insufficient. The mechanism of HDL-C elevation should be considered. The agents tested thus far may have increased HDL-C by forming large, cholesterol-rich HDL particles at the expense of medium- and small-HDL particles; having an overall null effect on total particle concentration. Indeed, there is evidence from 2D-gel electrophoresis that very high HDL-C levels observed in CETP deficiency result from a shift from small- and medium-HDLs to large-HDL particles [19]. Thus, HDL directed therapies – especially CETP inhibitors – might increase HDL-C without increasing the number of total HDL-P and possibly reducing the number of potentially beneficial medium-HDL particles. Considering that medium- and total HDL particle concentrations may represent superior predictors of cardioprotection, this hypothesis could explain the failures of the CETP inhibitors and niacin to prevent CVD. We speculate that HDL directed therapies might be more effective in reducing CVD-related events if the number of circulating HDL particles was increased by therapy, especially medium-HDLs.
In light of recent research showing that certain subspecies of HDL (such as medium-HDL and HDL-3) may specifically contribute to cardioprotection, it is our opinion that the focus of research and potential therapies should shift to these promising targets. Of particular interest is the protein cargo of these HDL subspecies, which may reveal important mechanisms related to their cardioprotective properties. For instance, HDL-3 is closely associated with PON1 enzyme activity [20], which is associated with cardioprotection [21, 22]. Notably, the cardioprotective association of HDL-3 was in part independent of both PON1 activity and HDL-C, indicating that there were unmeasured predictive elements of the HDL-3 proteome; these may be apolipoproteins, or ancillary proteins that are specifically associated with HDL-3 [17].
In summary, it is our opinion that the recent failure of increased HDL-C to be cardioprotective likely reflects the fact that increasing HDL-C alone does not adequately increase the concentration or activity of cardioprotective HDL subspecies. It would be an error to say that studies of HDL-C demonstrate that HDL is not cardioprotective. Increased total HDL particle concentration, or perhaps a specific increase in medium-HDL particles, may confer greater protection against CAAD and CHD than pharmaceutically generating a preponderance of large, cholesterol-rich HDL particles. Future research should focus on narrowing down focus through computational, structural and functional studies to identify the specific molecule or molecules that are responsible for the expected cardioprotective effect of HDL.
References
1. Castelli WP. Cardiovascular disease and multifactorial risk: challenge of the 1980s. Am Heart J. 1983; 106: 1191–1200.
2. Gordon DJ, Rifkind BM. High-density lipoprotein–the clinical implications of recent studies. N Engl J Med. 1989; 321: 1311–1316.
3. Rye KA, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2008; 50: S195–S200.
4. Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005; 85: 1343–1372.
5. Barter PJ, Barter PJ, Caulfield M, Caulfield M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007; 357: 2109–2122.
6. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012; 367: 2089–2099.
7. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011; 365: 2255–2267.
8. Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012; 380: 572–580.
9. Shen BW, Scanu AM, Kézdy FJ. Structure of human serum lipoproteins inferred from compositional analysis. Proc Natl Acad Sci U S A. 1977; 74: 837–841.
10. Huang R, Silva RAGD, Jerome WG, Kontush A, et al. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol. 2011; 18: 416–422.
11. Rosenson RS, Brewer HB, Chapman MJ, Fazio S, et al. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011; 57: 392–410.
12. Asztalos BF, Sloop CH, Wong L, Roheim PS. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta 1993; 1169: 291–300.
13. Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin lab. 2002; 48: 171–180.
14. Asztalos BF, Cupples LA, Demissie S, Horvath KV, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004; 24: 2181–2187.
15. Harchaoui El K, Arsenault BJ, Franssen R, Després J-P, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009; 150: 84–93.
16. Mackey RH, Greenland P, Goff DC, Lloyd-Jones D, et al. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2012; 60: 508–516.
17. Kim DS, Burt AA, Rosenthal EA, Ranchalis JE, et al. HDL-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. J Am Heart Assoc. 2014; 3: e000902.
18. Hutchins PM, Ronsein GE, Monette JS, Pamir N, et al. Quantification of HDL particle concentration by calibrated ion mobility analysis. Clin Chem. 2014; 60: 1393–1401.
19. Asztalos BF. Apolipoprotein composition of HDL in cholesteryl ester transfer protein deficiency. J Lipid Res. 2003; 45: 448–455.
20. Kontush A, Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol. 2003; 23: 1881–1888.
21. Jarvik GP, Rozek LS, Brophy VH, Hatsukami TS, et al. Paraoxonase (PON1) Phenotype Is a Better Predictor of Vascular Disease Than Is PON1192 or PON155 Genotype. Arterioscler Thromb Vasc Biol. 2000; 20: 2441–2447.
22. Kim DS, Marsillach J, Furlong CE, Jarvik GP. Pharmacogenetics of paraoxonase activity: elucidating the role of high-density lipoprotein in disease. Pharmacogenomics 2013; 14: 1495–1515.
The authors
Daniel Seung Kim1–3† BS; Patrick M. Hutchins4† PhD; Gail P. Jarvik1,2 MD, PhD
1Department of Genome Sciences, University of Washington, Seattle, WA, USA
2Division of Medical Genetics, Department of Medicine, University of Washington, Seattle, WA, USA
3Department of Biostatistics, University of Washington, Seattle, WA, USA
4Division of Metabolism, Endocrinology, and Nutrition, Department of Medicine, University of Washington, Seattle, WA, USA
†Authors contributed equally to this work
*Corresponding author
E-mail: pair@u.washington.edu
Acknowledgement
DSK is supported in part by 1F31MH101905-01 and a Markey Foundation Award. PMH is supported by a Cardiovascular Fellowship Training Grant (NIH T32HL007828). Work on the CLEAR study referenced within was supported by National Institutes of Health RO1 HL67406 and a State of Washington Life Sciences Discovery Award (265508) to the Northwest Institute of Genetic Medicine.