The relevance of the manufacturer in indirect immunofluorescence standardization
Autoantibody detection is a powerful laboratory tool for clinical diagnosis in the autoimmune diseases field. Among the techniques most widely used worldwide, indirect immunfluorescence (IFA) plays a particularly important role not only in the diagnosis but in the follow up of many diseases and remains the hallmark despite the introduction of new techniques in the routine of clinical laboratories. Witness to this is the renaissance of the antinuclear antibodies (ANA) screening on HEp2 cells by this techique or the renewal of the detection of anti-endomysium antibodies on monkey esophagus as the gold standard serological test for celiac disease. Therefore, IFA is a technique in full validity and requires a level of standardization that unfortunately is far from being achieved.
by Petraki Munujos, PhD
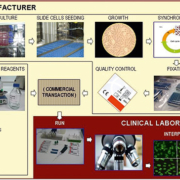
The efforts to improve standardization of indirect immunofluorescence as a diagnostic tool are numerous worldwide. Traditionally, the players involved in standardization have been clinical laboratories, clinicians, regulators, and to a lesser degree, diagnostic reagents manufacturers. Energy has been concentrated basically in aspects like the control of laboratory procedures, unification of nomenclatures and classifications, guidelines on how to report the results, preparation of recommendations, definition of diagnostic criteria and diagnostic algorithms and development of external quality control programs. In these iniatives, laboratory staff, clinicians and regulators are mainly involved. Nevertheless, those aspects regarding the design, development and manufacturing of the reagents, which involve manufacturers, are basically ignored. And this is probably due to the fact that the evolution of the technology has led to a truncated view of the test procedure resulting in a misconception of what needs to be standardized. In other words, the execution of many procedures is nowadays being shared between the manufacturer, who actually initiates the assay, and the laboratory, where the test is finalized. In old scientific articles related to ANA, the Material and Methods section usually started with the cell culture, the preparation of the slides and the fixation among others, and the sample incubation was only one more step of the whole procedure. Currently, the Material and Methods section starts with the sample preparation and instead of describing all the preliminary steps, one can find the name and references of the manufacturer. Figure 1 illustrates what would be the whole test procedure, showing the part performed in the clinical laboratory, actually the only part which is taken into consideration when dealing with standardization. So, to ensure appropriate use of indirect immunofluorescence testing, clinicians, diagnostic laboratories, regulators and reagents manufacturers should be involved and share the tasks of identifying and managing the key points leading to proper results.
Evidences of disparity
At the level of the manufacturer, the potential variability in the performance of the kits lies in features like the reagents and materials that are purchased or manufactured to become components of the kit, the procedures and conditions of manufacturing (fixatives, temperatures, formulations), the reliability of the serum samples used to set up the calibration of the determination (basically, the sample dilution which actuallly acts as the cut-off point), and the stability of the final product (1).
When approaching the participation of the manufacturer in the standardization of antibody testing, it is observed that what basically matters for industry is the standardization of the manufacturing processes. This normally occurs in an environment of Quality System Certifications, like GMP, ISO-9001 or ISO-13485 and under the requirements of the European Directive on In Vitro Medical Devices, and it is strengthened by the manufacturer’s own interest in having robust and reliable processes. Nevertheless, despite regulatory compliant and well implemented standardized processes, there are several aspects that make final reagents differ from one manufacturer to another. Below are reviewed some examples of variation on the results depending on the manufacturer source.
Dense fine speckles 70 (DFS70) antigen
As with other fluorescence patterns, the typical DFS pattern (lens epithelium-derived growth factor) can vary depending on the manufacturer source of the HEp2 slides used. The variations consist basically in different sensitivities and even in positive and negative results for the same sample run in different slide brands. Inconsistencies are also observed when comparing fluorescence with the results obtained by means of ELISA (2,3).
Ribosomal P protein (Rib P)
In studies performed by Mahler et al. (4) to determine the sentitivity of the immunofluorescence technique to detect antibodies against ribosomal P protein, several different HEp2 slides manufacturers were used, resulting in significant differences in patterns of staining for monospecific anti-Rib-P sera. Differing patterns were observed for the same sample, from a fine speckled nucleoplasmic pattern, to a diffuse cytoplasmic staining, or a fine speckled cytoplasmic pattern.
CDC/AF Reference Human Sera
When running reference sera on HEp2 slides coming from different manufacturers, variations of unknown origin can be observed. While most brands produce the expected specific pattern, there are often differences among brands like the ones shown in Figure 2.
Labile nuclear antigens
Most of the patterns observed when analysing the presence of ANA in patients sera by IFA on HEp2 cells slides are suitably detected in most slides brands. However, there are some antigens for which expression may significantly vary from one manufacturer to another like Jo1, PCNA or SSA/Ro (5). These antigens are not always well preserved in the substrates and they can be extremely sensitive to handling, to certain fixatives and in some cases, they can be just washed out during the manufacturing process, resulting in a poor presence or a total lack of antigenic molecules available to capture the antibody being analysed.
Antineutrophil cytoplasmic antibodies (ANCA)
The neutrophil substrates used in the detection of ANCA may vary in their ability to give the typical immunofluorescence patterns described and established by consensus groups, i.e. a diffuse granular cytoplasmic staining with higher interlobular intensity (C-ANCA), a compact staining of the perinuclear zone of the cytoplasm (P-ANCA) and a broad non homogeneous perinuclear staining, eventually accompanied by a diffuse cytoplasmic pattern with no accentuation of the interlobular zone (X-ANCA). In general, substrates differ in their ability to distinguish between a C-ANCA and X-ANCA. In a study by Pollock et al. (6), it was observed that although all commercial neutrophil substrates consistently demonstrated nuclear extension of perinuclear fluorescence with sera containing P-ANCA with MPO specificity, there were more problems in P-ANCA testing than in C-ANCA, due basically to the eventual presence of additional cytoplasmic fluorescence.
Crithidia luciliae
In a similar way as observed in HEp2 cells immunofluorescence patterns, the anti-nDNA test on Crithidia luciliae slides may show significant differences among manufacturers. The variety of strains available in cell banks contribute to the heterogeneity of results. Apart from the kinetoplast, other organelles can be stained by antibodies from the sample, like the nucleus, the basal body and the flagellum. Depending on the conditions of preparation of C. luciliae substrates and on the nature of the sample analysed, different patterns of stained organelles can be observed. Nevertheless, the only specific staining to be considered as a positive result is the kinetoplast staining. In addition to anti-nDNA antibodies, there are other antibodies in the serum of lupus patients that can react with the substrate. The so called anti-nucleosome antibodies are antibodies that react with histones exposed in the nucleosome. It is well known that treating C. luciliae substrate with HCL eliminates histone from the kinetoplast (7). This could be another point of possible discrepancy among manufacturing processes if some include the histone removal procedure and some others do not. Furthermore, the cell cycle of C. luciliae may influence histone appearance in the kinetoplast. Therefore, the manufacturing process of C. luciliae slides, including culture, harvest, fixation and drying, can cause variation in the results.
Aspects providing variablity
Among the players participating in autoimmune diagnostics, there is no doubt that manufacturers hold the know-how of preparing diagnostic kits and are the true experts in the development of test methods. However, despite the standardized manufacturing processes and the CE-certifications or FDA approvals, there are several aspects that are found to be sources of variabilty. These aspects should be addressed and recommendations on key points should be created by specialized committees with the participation of laboratory experts, clinicians and manufacturers. The definition and control of the raw materials incorporated in the kit production is a common and regulated practice in any kind of manufacturing process. But recommendations on nature, compostion or quality grades of key materials, including culture media, cell type and strain or fluorescent conjugates is still lacking. In the case of tests based on cellular substrates, extracellular matrix (ECM) proteins are commonly used to aid the spreading and growth of cells on the slide glass surface. Many ECM proteins contain defined amino acid sequences to which cell surface integrin receptors bind specifically. ECM, together with growth factors in the culture medium, work to produce an appropriate in vitro proliferative response, promoting cell growth and spreading. Altering cell-ECM contacts results in coordinated changes in cell, cytoskeletal, and nuclear form. Thus, the choice of the right ECM to coat the glass slides used as growing surface deserves our attention since it might have a direct effect on the fluorescent pattern finally observed (8). It is also common to use synchronization agents to achieve a greater rate of mitotic cells. Due to the fact that these compounds may be toxic for the cell, some cell disturbances may occur that can impact the morphology or the behaviour of the final cell preparation.
Diagnosis by means of tissue sections remains very important in liver autoimmune diseases like autoimmune hepatitis (AIH) or primary billiary cirrhosis (PBC). In particular, the detection of anti-smooth muscle antibodies (ASMA), antibodies to liver-kidney microsomes (LKM antibodies) and anti-mitochondrial antibodies (AMA) are considered important diagnostic tools. Only a few guidelines have been published on the obtention of tissue sections (9), while the variations in the preparation of tissue blocks regarding orientation, preservation conditions, and sectioning keep on contributing to the heterogeneity of results, especially in the case of tissues that are not morphologically homogeneous. For instance, the LKM antibodies can only be well defined if the kidney section has the proper orientation that allows the distinction between proximal and distal renal tubules and, thus, between LKM and AMA.
Considering that the expression and topographical distribution of autoantigens is under the direct influence of the HEp-2 fixation method, some immunofluorescence patterns are not adequately expressed due to the way that the antigenic substrate is prepared. This aspect equally affects tissue and cell substrates. As for the sensitivity of the tests, differences among manufacturers are due to the use of fixatives to prolong shelf-life. The use of slides without fixation seems to be the best choice for most autoantibody patterns. Nevertheless, there are several staining patterns that need the substrate to be fixed (figure 3), like anti-islet cells antibodies or anti-adrenal cortex antibodies.
A less frequent but significant source of variability in the immunofluorescence on tissue sections can be found in the origin of the animal used (Figure 4). Definition of suitable species and strains should be addressed in some cases in which the levels of antigen expression may differ. This affects the sensitivity of the test, especially in samples with moderate or low titers of antibody.
Considering the complexity and diversity of manufacturing processes and subprocesses and their impact on the final test performance, it is important to combine the efforts of laboratory experts, clinicians and manufacturers in the task of standardizing those key aspects that could otherwise keep on undermining the successful harmonization of the results obtained in the clinical laboratory.
References
1. Fritzler MJ, Wiik A, Fritzler ML, Barr SG. The use and abuse of commercial kits used to detect autoantibodies. Arthritis Res Ther 2003, 5:192-201
2. N.Bizzaro, E.Tonuttiand D.Villalta, «Recognizing the dense fine speckled/lens epithelium-derived growth factor/p75 pattern on HEP-2 cells: not an easy task! Comment on the article by Mariz et al,» Arthritis Rheum, vol. 63, no. 12, pp. 4036-4037, 2011
3. Mahler M. The clinical significance of anti-DFS70 antibodies as part of ANA testing. In: K. Conrad, E.K.L. Chan, M.J. Fritzler, R.L. Humbel, P.L. Meroni, G. Steiner, Y. Shoenfeld (Eds.). Infection, Tumors and Autoimmunity, AUTOANTIGENS, AUTOANTIBODIES, AUTOIMMUNITY, Volume 9, p.342-350. PABST, 2013.
4. Mahler M, Ngo JT, Schulte-Pelkum J, Luettich T, Fritzler MJ. Limited reliability of the indirect immunofluorescence technique for the detection of anti-Rib-P antibodies. Arthritis Research & Therapy 2008, 10:R131
5. Dellavance A, de Melo Cruvinel W, Carvalho Francescantonio PL, Pitangueira Mangueira CL, Drugowick IC, RodriguesSE; Coelho Andrade LE. Variability in the recognition of distinctive immunofluorescence patterns in different brands of HEp-2 cell slides J Bras Patol Med Lab 2013;49( 3):182-190.
6. Pollock W, Clarke K, Gallagher K, Hall J, Luckhurst E, McEvoy R, Melny J, Neil J, Nikoloutsopoulos A, Thompson T, Trevisin M, Savige J. Immunofluorescent patterns produced by antineutrophil cytoplasmic antibodies (ANCA) vary depending on neutrophil substrate and conjugate. J Clin Pathol 2002;55:680–683
7. Kobkitjaroen J, Jaiyen J, Kongkriengdach S, Potprasart S, Viriyataveekul R. Comparison of Three Commercial Crithidia luciliae Immunofluorescence Test (CLIFT) Kits for Anti-dsDNA Detection. Siriraj Med J 2013;65:9-11
8. (Integrin Binding and Cell Spreading on Extracellular Matrix Act at Different Points in the Cell Cycle to Promote Hepatocyte Growth Hansen LK,. Mooney DJ, Vacanti JP, Ingber DE. Molecular Biology of the Cell 1994;5:967-975
9. Vergani D, Alvarez F, Bianchi FB, Cançado ELR, Mackay IR, Manns MP, Nishioka M, Penner E. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. Journal of Hepatology 2004;41: 677–683