Therapeutic drug monitoring of favipiravir, a potential COVID-19 drug candidate
The COVID-19 outbreak caused by severe acute respiratory syndrome virus 2 (SARS-CoV-2) that was declared a pandemic in March 2020 has resulted in a the development of diagnostic tests and vaccines at an unprecedented speed. The development of de novo therapeutics, though, takes considerably longer, which is where the repurposing of existing drugs is being used to investigate efficacy against COVID-19. This article discusses a new method for the therapeutic monitoring of one potential COVID-19 drug candidate, favipiravir, in plasma.
Introduction
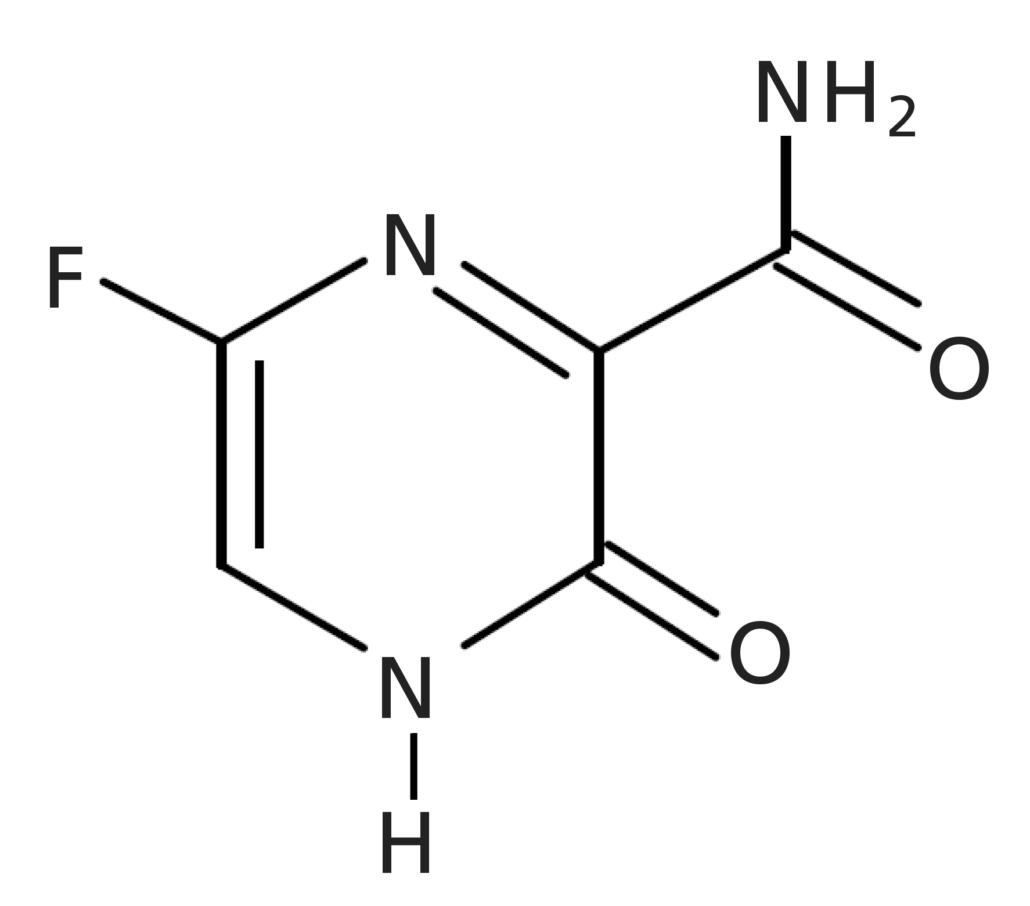
In 2020, owing to the global health crisis caused by the spread of the coronavirus (COVID-19), the urgency of fast and efficient development of both new pharmaceuticals for treatment as well as vaccines became utterly apparent. Favipiravir (Fig. 1; brand name: Avigan®), a promising drug candidate for COVID-19, is classified as an anti-influenza drug, evaluated and developed for both novel and re-emerging influenza viruses [1,2]. Favipiravir undergoes renal excretion, and is eliminated in the urine mainly as a hydroxide. To ensure safety and efficacy of the amount of drug taken, accurate monitoring of drug levels is crucial but challenging, owing to its once daily dosing regimen [3].
This article introduces a novel approach for the highly sensitive, quantitative determination of favipiravir in plasma using a simple high-pressure liquid chromatography (HPLC) set-up with fluorescence detection.
Sample preparation
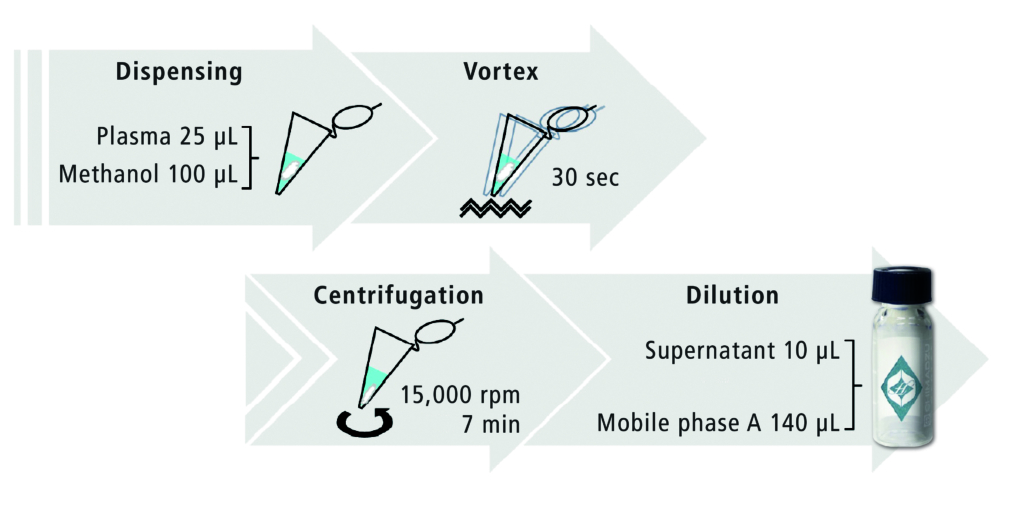
Plasma and serum samples typically require deproteinization to reduce matrix interferences and prevent clogging and degradation of the analytical column. In this study, deproteinization was performed as depicted in Figure 2. Twenty-five microlitres of plasma were added to 100 μL of methanol, mixed well and then centrifuged. The supernatant was diluted 15-fold with mobile phase before HPLC analysis.
Analytical method
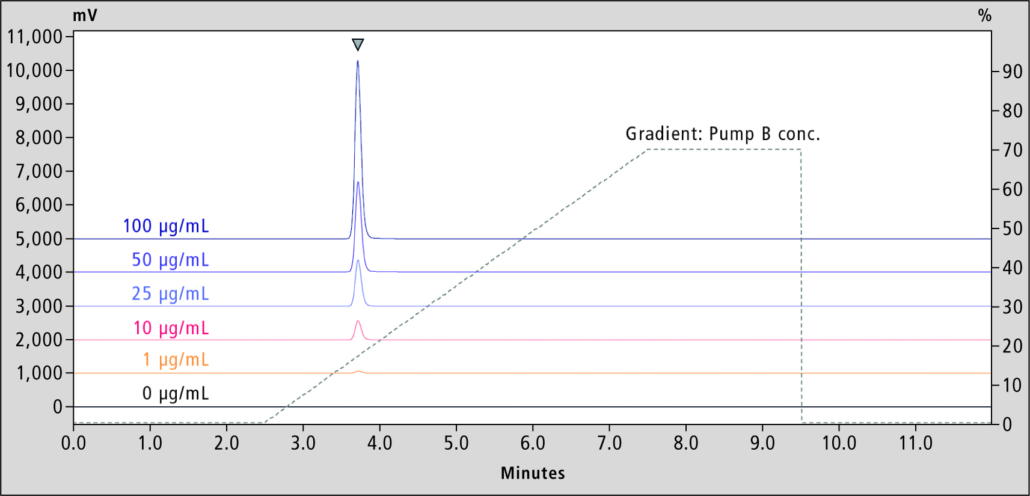
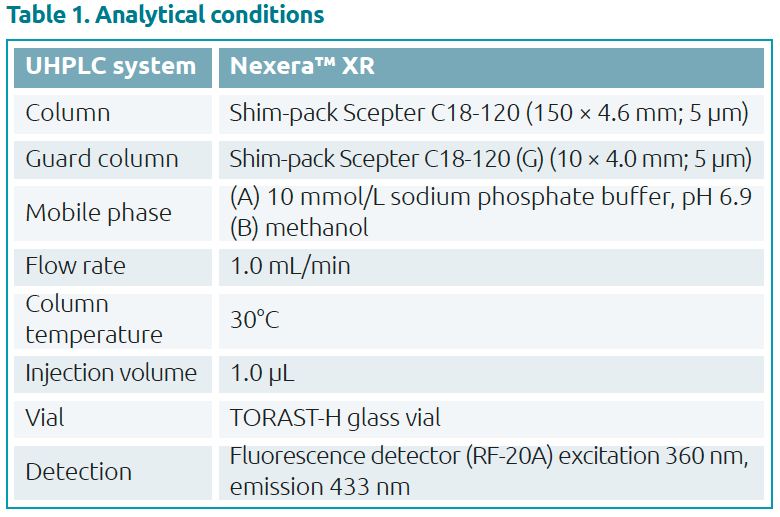
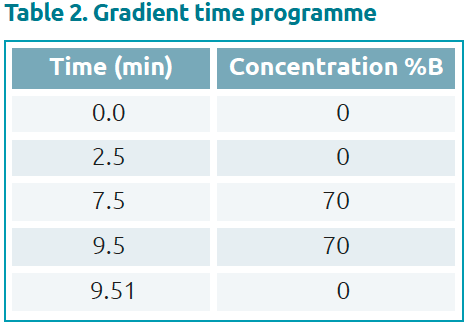
Favipiravir standard was purchased from Alsachim, France. The calibration curve and quality control (QC) samples were prepared by adding favipiravir to normal human plasma. Measurement was performed by HPLC using the analytical method shown in Tables 1 and 2. Favipiravir was analysed on a Shim-pack Scepter C18-120 separation column fitted with a matching guard column. A calibration curve was generated from analysis of favipiravir standard solutions with final concentrations of 1, 10, 25, 50 and 100 μg/mL (n=6) in plasma. Resulting chromatograms are shown in Figure 3.
Calibration
In this concentration range, good linearity for the determination of favipiravir in plasma was obtained with a correlation coefficient R2=0.999. Precision in n=6 samples was determined as percentage of relative standard deviation (% RSD) of 0.21–0.31%, and accuracy of 92.1–106%, which was within the acceptance limits of 100±8.0%.
Validation test with QC samples
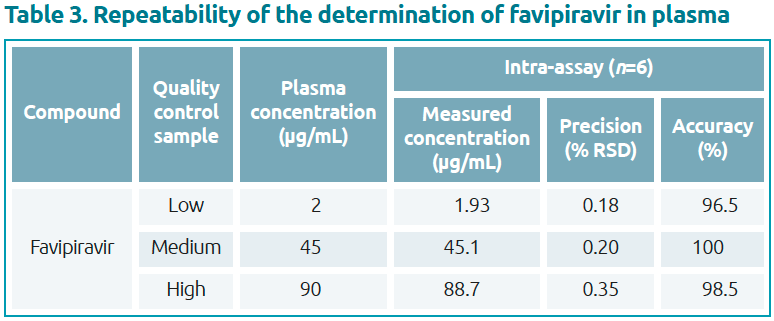
A validation test was performed using favipiravir at final concentrations of 2, 45 and 90 μg/mL in plasma (Table 3). The validation test resulted in precision (% RSD from n=6) between 0.18% and 0.35%, with accuracy of 96.5–100%, which met the acceptance limits of 100±4.0%.
Conclusion
This article demonstrates a novel approach for therapeutic drug monitoring of a COVID-19 drug candidate. A simple, sensitive, quantitative HPLC method for the determination of favipiravir in plasma was developed. It was shown to provide good accuracy and precision over a wide concentration range using a standard fluorescence detector.
Acknowledgment
The source for all Figures and Tables in this article is Shimadzu.
Figure 1. Structure of favipiravir
Figure 2. Deproteinization protocol
Figure 3. Standard chromatograms and gradient profile for the HPLC analysis of favipiravir in plasma
The authors
Gesa Johanna Schad1 PhD, Yusuke Osaka2 MSc, Risa Suzuki2 PhD
1Shimadzu Europa, Duisburg, Germany
2Shimadzu, Kyoto, Japan
References
- Gowen BB, Sefing EJ, Westover JB, et al. Alterations in favipiravir (T-705) pharmacokinetics and biodistribution in a hamster model of viral hemorr-
hagic fever. Antiviral Res 2015; 121: 132–137 (https://bit.ly/30OSHVh). - Takashita E, Ejima M, Ogawa R, et al. Antiviral susceptibility of influenza viruses isolated from patients pre- and post-administration of favipiravir. Antiviral Res 2016; 132: 170–177 (https://bit.ly/3sod9YA).
- Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther 2020; 209: 107512 (https://bit.ly/3ej3nyy).
- Suzuki R, Osaka Y. Application news No. L570: Quantitative analysis of favipiravir spiked in plasma by HPLC. Shimadzu Corporation 2020, August (https://bit.ly/3EoLSHM)