Thiopurine methyltransferase: a paradigm of pharmacogenetics
Thiopurine methyltransferase (TPMT) is an enzyme involved in the metabolism of thiopurine drugs such as azathioprine. TPMT genotyping or measurement of enzyme activity prior to treatment enables prediction of those individuals most at risk of myelotoxicity in whom these drugs should be avoided or used in reduced doses.
by Dr Joshua Ryan, Christiaan Sies and Associate Professor Chris Florkowski
Background
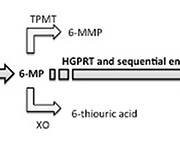
Thiopurine drugs azathioprine (AZA) and 6-mercaptopurine (6-MP) are widely used as steroid-sparing agents in autoimmune diseases such as inflammatory bowel disease (IBD), autoimmune hepatitis, rheumatoid arthritis as well as following organ transplantation and in leukaemia [1, 2]. In the body, AZA is rapidly converted, largely non-enzymatically, into 6-MP which is subsequently metabolized to the active compound 6-thioguanine (6-TG) by several sequential enzymes starting with hypoxanthine guanine phosphoribosyltransferase (HGPRT) (Fig. 1) [1].
6-TG is a nucleotide analogue that is incorporated into DNA and RNA thereby interfering with cellular function and protein synthesis and also blocks the cellular signalling protein Rac 1, resulting in T cell apoptosis [3]. Although these mechanisms underlie the therapeutic action of thiopurine drugs, excessive and toxic levels of 6-TG however, can cause myelosuppression and life-threatening leukopenia [3]. Two additional enzymes xanthine oxidase (which normally converts xanthine to uric acid and is inhibited by the gout treatment drug allopurinol) and thiopurine methyltransferase (TPMT; an enzyme whose natural substrate is unknown) play important roles in thiopurine drug metabolism, providing alternative pathways that convert 6-MP into the inactive metabolites 6-thiouric acid and 6-methyl-mercaptopurine (6-MMP), respectively (Fig. 1) [1, 3].
Any factors that reduce the activity of these pathways, either acquired (e.g. allopurinol) or inherited (e.g. TPMT deficiency) can result in a greater proportion of the drug being metabolized to active metabolites, conferring increased risk of toxicity.
TPMT deficiency, pathophysiology and clinical effects
The activity of this enzyme in the body is co-dominantly inherited and the TPMT gene is on chromosome 6 [4]. The majority of the population (89–92%) inherit two normally functioning copies of the gene (allele designated TMPT*1). A smaller proportion (8–11%) inherit one deficiency allele (of which 29 have been identified) giving low TPMT activity and rarely, 0.3% of individuals are homozygous for the deficiency alleles resulting in virtually no TMPT activity [1, 3].
The TPMT*3A variant is a double mutant allele (G460A on exon 7 and A719G on exon 10), TPMT*3C has the same A719G mutation on exon 10 mutation as for TPMT*3A and TPMT*2 has a G238C point mutation on exon 5 [3]. Together, alleles TPMT*2, *3A and *3C account for the majority (up to 95%) of deficiency alleles that are seen in Caucasian, Asian and African-American populations [3, 5]. The resulting trimodal distribution of enzyme levels (low, intermediate, normal) was demonstrated in our local population in a study of 407 patient samples referred to Canterbury Health Laboratories from throughout New Zealand over a 2-year period (Fig. 2) [6]. Patients with reduced red blood cell (RBC) TPMT activity below 12units/mL RBCs went on to have TPMT genotyping for three common deficiency alleles (*2, *3A, *3C) [6]. The three groups of patients identified had enzyme activity that reflected the underlying genotype (i.e. normal TPMT, deficiency allele heterozygotes and deficiency allele homozygotes) although there was some overlap in enzyme activities seen between the normal and heterozygous deficiency patients (Fig. 2).
Patients with inherited TPMT deficiency (i.e. homozygous for deficiency alleles) are at high risk of potentially fatal myelosuppression, as in these patients a greater proportion of the thiopurine drug is metabolized to active metabolites resulting in bone marrow toxicity. In these patients AZA should be avoided or the dose markedly reduced [1, 3]. Heterozygotes for deficiency alleles also have increased rates of adverse effects and in these patients, some dose reduction (by 50–67%) is recommended [3].
Enzyme activity measurement
TPMT enzyme activity may be measured in RBCs prior to commencing the thiopurine drugs to identify TPMT status (‘phenotype’) and to help guide drug and dose decisions. Traditional enzymatic methods used a 6-MP substrate and radiolabelled methyl donor and the radiolabelled product (6-MMP) was measured by scintillation counting [1, 6]. More recent methods have avoided the use of radiolabelled compounds by using HPLC-UV or mass spectrometry for product detection [3]. Patients found to have low levels on phenotyping may be confirmed using genetic testing of TPMT. In-house studies on a local population found TPMT activity levels ≥10.7 units/mL RBC excluded the presence of a deficiency allele (normal reference interval 9.3–17.6 units/mL RBC) [6]. It should be noted, however that reporting units may vary between laboratories (e.g. reported per mL of RBC or per g of Hb to adjust for variations in hematocrit) and results should be interpreted relative to lab-specific reference intervals [4].
Measurement of RBC TPMT enzyme activity, however, has some important caveats. For example, TPMT activity is not reliable in patients who have received a blood transfusion in the preceding 120 days (i.e. RBC average life span) due to interference from donor RBC TPMT [3]. Chronic renal failure may elevate TPMT activity and higher levels are seen in pre-dialysis samples compared with those collected following dialysis [1,4]. Differences have also been reported with other diseases (e.g. IBD, autoimmune hepatitis, pemphigus) but the differences are small and clinically unimportant [4]. Various drug compounds can affect TPMT activity causing a decrease (e.g. sulfasalazine) or increase (e.g. methotrexate, trimethoprim) of in vitro activity however, in assays with a RBC wash step the interfering compounds may be largely removed [4].
In our laboratory, samples are collected in EDTA whole blood and stored refrigerated (or chilled with an ice pack for transport). A previous local study of samples referred to Canterbury Health Laboratories over 8 years (n=6348) found delay in analysis had a small but significant reduction on TPMT activity of 0.26 units/mL RBC for each additional day prior to analysis [2]. Another published review found TMPT activity to be relatively stable at room temperature or refrigerated for 7 days, although may be reduced in some disease states such as leukaemia [4].
The role of genotyping
Genotyping may also be used to assess TPMT status prior to commencing AZA or 6-MP either alone or commonly to confirm low enzyme activity. It has the advantage of not being affected by blood transfusions and is less affected by pre-analytical factors. The limitation is that only common deficiency alleles are tested for (e.g. TPMT*2, *3A, *3C) and rare deficiency alleles will be missed using genotyping alone [1]. Enzyme activity measurement may also identify those patients who have ultra-high TPMT activity (up to 2% of patients) and who may require higher than usual doses, information that routine genotyping alone would not provide [3].
The place of 6-TG monitoring and other investigations
Once treatment has been initiated (ideally following TPMT assessment), ongoing treatment is usually monitored by measurement of the metabolite 6-TG, for which therapeutic ranges have been suggested – for example RBC 6-TG >235 pmol/8×108 cells is associated with maximum efficacy in IBD [3]. The metabolite 6-MMP is usually monitored together with 6-TG in order to differentiate non-compliance or inadequate dose (i.e. low 6-TG and 6-MMP), from those patients where AZA is preferentially metabolized to 6-MMP (i.e. high 6-MMP and low 6–TG) [3]. In the latter scenario, increasing the dose can lead to further elevation of RBC 6-MMP and values >5700 pmol/ 8×108 cells are associated with hepatotoxicity [3].
All of the above, however does not obviate the need for patients on thiopurine drugs to undergo regular monitoring of routine blood tests (e.g. full blood count, liver function tests) to allow early detection of the most serious adverse effects of myelosuppression and liver dysfunction [3, 5]. This monitoring in required regardless of TPMT status and especially given that TMPT deficiency does not predict all cases of myelosuppression in patients on these medications [3].
Recommended ractice
The US Food and Drug Administration (FDA) and prescribing information for AZA and 6-MP recommend assessing TPMT status with genotyping or phenotyping prior to starting treatment with thiopurine drugs [7]. There is, however considerable variation in what guidelines recommend in routine practice regarding TPMT and indeed, some systematic reviews have concluded that there is insufficient evidence for TPMT testing in chronic inflammatory diseases [5]. Based on a frequency of 1 in 300 for homozygous deficiency, we calculated that in New Zealand with an assay cost of $57.75 per assay (in 2005), this would equate to a cost of $17,250 per potentially fatal episode averted [6]. Similar calculations in the United Kingdom have indicated that the costs per quality adjusted life-year (QALY) gained are well within acceptable thresholds [3].
Future developments and conclusion
As with many other drugs, the metabolism of AZA and 6-MP is complex and information is accumulating on other inherited and acquired factors contributing to the adverse effects of these medications. For example, inherited differences in other enzymes involved in their metabolism such as xanthine oxidase and inosine triphosphate pyro-phosphohydrolase (ITPase) may have a role and further study on these mechanisms is required [3].
In conclusion, TPMT provides a paradigm where testing that provides information about the genetic makeup of an individual (directly or indirectly through enzyme testing) enables important guidance on drug dosing, with the potential to avoid serious side-effects. Measuring TPMT activity in RBCs prior to starting AZA or 6-MP (with confirmatory genotyping) is a simple yet effective way to detect those with TPMT deficiency who are at particular risk of bone marrow toxicity, although ongoing monitoring with routine bloods is required in all patients to identify serious drug adverse effects.
References
1. Gearry RB, Barclay ML, et al. Thiopurie methyltransferase and 6-thioguanine nucleotide measurement: early experience of use in clinical practice. IMJ 2005; 35: 580–585.
2. Van Egmond R, Barclay ML, et al. Preanalytical stringency: what factors may confound interpretation of thiopurine S-methyl transferase enzyme activity. Ann Clin Biochem. 2013; 50: 479–484.
3. Ford LT, Berg JD. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J Clin Pathol. 2010; 63: 288–295.
4. Loit E, Tricco AC, et al. Pre-analytic and analytic sources of variation in thiopurine methyltransferase activity measurement in patients prescribed thiopurine-based drugs: a systematic review. Clin Biochem. 2011; 44: 751–757.
5. Booth RA, Ansari MT, et al. Ann Int Med. 2011; 154: 814–823.
6. Sies C, Florkowski C, et al. Measurement of thiopurine methyl transferase activity guides dose-initiation and prevents toxicity from azathioprine. NZMJ 2005; 118: U1324.
7. Nguyen CM, Mendes MAS, Ma JD. Thiopurine Methyltransferase (TPMT) genotyping to predict myelosuppression risk. PLoS Curr. 2011; 3:RRN1236.
The authors
Joshua Ryan MB MAACB FRCPA, Christiaan Sies MSc, Chris Florkowski* MD MRCP(UK) FRACP FRCPA
Canterbury Health Laboratories, Christchurch, New Zealand
*Corresponding author
E-mail: Chris.florkowski@cdhb.health.nz