TNF Receptors – powerful biomarkers for detecting diabetic kidney disease a decade in advance
Kidney disease is one of the most life-threatening complications of diabetes and as the global incidence of diabetes soars, largely due to the dramatic increase in type 2 diabetes (T2DM), there will be a seismic shift in the number of patients in need of treatment through dialysis or transplant. Since up to 40% of diabetic patients develop symptoms of diabetic kidney disease (DKD), accurate and early identification of which patients are at the highest risk of progression from DKD to end stage renal disease (ESRD) will enable early initiation of protective renal therapies with subsequent reduction in healthcare costs and improved patient outcomes.
The cytokine TNFα, part of the Tumour Necrosis Factor (TNF) superfamily that plays a key role in homeostasis, has been implicated in the pathogenesis of diabetic kidney disease for over 20 years [1]. Researchers conclude that the elevated levels seen in diabetic patients could be the result of a TNFα driven dysregulation of the inflammatory/apoptotic pathways, which leads to kidney injury. The spotlight has recently shifted onto the TNF α receptors, Tumour Necrosis Factor Receptor 1 (TNFR1) and Tumour Necrosis Factor Receptor 2 (TNFR2), after a number of studies showed how elevated levels of these proteins were a predictor of progressive kidney disease.
In this article we look at the development of an In-Vitro Diagnostic test (IVD), the ‘EKF sTNFR1 Test’. This has been developed by EKF Diagnostics to measure levels of TNFR1 in plasma or serum in light of scientific evidence that this robust biomarker provides valuable prognostic information for diabetic patients at risk of progressive renal decline and ESRD.
The scientific evidence for the involvement of TNF receptors in kidney disease
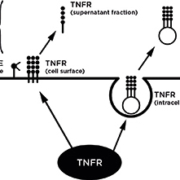
Cytokine TNFα is a transmembrane protein generated by many cells, including lipocytes, endothelial cells and leukocytes. After processing by TNFα-converting enzyme (TACE), the soluble form of TNFα is cleaved from transmembrane TNFα and mediates its biological activities through binding the receptors TNFR1 and TNFR2 either in their transmembrane or soluble forms to activate inflammatory and stress response pathways (Figure 1). Transmembrane TNF-α also binds to TNFR1 and TNFR2 so that both transmembrane and soluble TNF-α can mediate downstream signalling events (apoptosis, cell proliferation and cytokine production).
In 2009, at the Joslin Diabetes Center, USA (the world’s largest diabetes research centre and an affiliate of the Harvard Medical School), researchers found that the presence of circulating soluble TNF receptors (sTNFR1 and sTNFR2) were strongly correlated with decreased renal function, or glomerular filtration rate (GFR). The research threw up questions about why these soluble receptors were indicative of renal disease. Were they playing an active part in causing disease, or were they just the by-product of the process? Elevations in circulating sTNFR1 have previously been reported in a wide variety of clinical conditions including cancer, congestive heart failure, rheumatoid arthritis, neurological diseases and infection; so what was their role in kidney disease?
Interestingly, as Niewczas et al. [2] pointed out, the decline of renal function was occurring in T1DM patients who had normal albumin excretion levels. This gave a clue to the researchers that the concentrations of these receptors were not merely markers of the injury leading to ESRD but were also involved in the inception of renal function decline, playing a part in inflammation and apoptosis.
1n 2012, the Joslin researchers published two further studies, on Type 1 and Type 2 diabetes cohorts, [3,4] and found that TNF receptor levels were robust predictors of progressive decline in GFR. The results showed that Type 1 Diabetes patients who had normal renal function at the onset, but TNFR2 levels in the highest quartile had a 60% cumulative incidence of reaching stage 3 Chronic Kidney Disease (CKD) with subsequent risk of progression to ESRD (compared to less than 20% in the lowest three quartiles) (Figure 2).
Most significantly, in Type 2 Diabetes patients with evidence of overt Kidney Disease (as evidence by elevated levels of albumin excretion levels) at the onset of the study, those with levels of TNFR1 in the fourth quartile had an 80% chance of developing renal disease over the twelve year period (compared to less than 20% of those in the lower three quartiles) (Figure 3).
These studies revealed that elevated TNF Receptor levels were a robust predictor of progressive disease in both Type 1 diabetes and Type 2 diabetes. In both studies, the levels of the TNFα levels also tended to predict progressive kidney disease, but less strongly than the TNF receptor levels. The data provided further evidence that inflammation in general, and the TNFα signalling pathway in particular, plays a role in kidney disease.
TNF receptors (TNFR1 and TNFR2) and their role in the disease process
So how are circulating TNFR receptors associated with early GFR reduction and kidney damage? It is known that the 55 kD TNFR1 and 75 kD TNFR2 receptors play a crucial part in apoptosis, survival and key aspects of the inflammatory and immune response. TNFR1 is abundant on all nucleated cells, but TNFR2 expression is restricted mainly to endothelial cells and leukocytes although this varies between normal and diseased tissues. Circulating TNFR1 in the plasma is released by two mechanisms: the inducible cleavage of the 34 kD TNFR1 extracellular domain by an enzyme known as ADAM17 and the constitutive release of a full-length 55 kD TNFR1 within exosome-like vesicles.
It is not-well understood whether the same mechanisms apply to TNFR2 release, or how this process is regulated and the biology of the soluble forms remain largely undiscovered. What is understood, however, is that in plasma, TNF receptors block TNFα from binding its target cell surface receptor and can therefore cause a prolonged and delayed effect of the cytokine. How subsequent damage occurs to the kidney is not well known, however sTNFRs have been shown to be involved in tubulointerstitial fibrosis, the characteristic tissue scaring that leads to kidney disease [5].
Seeing into the future: a powerful diagnostic test for DKD
The diagnosis of DKD is conventionally made by assessment of overall GFR and the presence of kidney damage is ascertained by either kidney biopsy or other markers of kidney damage such as microalbuminuria or proteinuria (collectively known as albuminuria – a condition where protein is lost in the urine). GFR is estimated in clinical practice using readily calculated equations that adjust serum creatinine values (measurement of the by-product of muscle metabolism cleared by the kidneys) to age, sex, and ethnicity. However, while laboratory tests which assess both serum creatinine and albuminuria are inexpensive and readily available, these parameters have a low predictive value.
In 2012, EKF Diagnostics signed an exclusive licence agreement for novel kidney biomarker technology that focused on sTNFR1 and sTNFR2. This was developed by a team led by Prof. Andrzej Krolewski, MD, PhD, Head of Section on Genetics and Epidemiology at the Joslin Diabetes Center, Professor of Medicine at Harvard Medical School. Prof. Krowlewski was recently awarded the American Diabetes Association’s 2014 Kelly West Award in Epidemiology for services to diabetes epidemiology.
EKF Diagnostics has worked in partnership with Joslin and other key diabetes research centres to further validate the clinical utility of the markers and develop its first IVD product, the sTNFR1 test kit. The sTNFR1 test is an easy-to-use, microtitre plate ELISA-based assay requiring minimal training, which uses standard laboratory equipment and monoclonal antibodies to analyse just 50 µL of blood serum or plasma. Accurate and reliable results are obtained in a few hours and the standard assay format means that the test requires minimal training.
Julian Baines, Group Chief Executive Officer of EKF Diagnostics highlights the benefits of the test, “Our new sTNFR1 test adds greatly to information provided by standard clinical tests and provides valuable long term prognostic information for progressive renal decline to ESRD with the potential to streamline diabetic patient management, reduce time and costs and improve patient outcomes.”
Further evidence for the use of sTNFRs for the early prediction of DKD
A number of high impact studies published this year have independently corroborated the original research by the Joslin Diabetes Center. This newly published data from eminent European research centres in France (SURDIAGNE Study Group) and Finland (FinnDiane Study Group) add to the expanding data set underpinning the value of sTNFR1/2 biomarkers.
In the FinnDiane cohort study of over 400 subjects with Type 1 Diabetes followed over an average of 9 years, researchers found that, “Circulating levels of sTNFR1 were independently associated with incidence of ESRD. This association was reported as both significant and biologically plausible and demonstrated added value of sTNFR1 as a biomarker” [6].
In France, Saulnier et al. [7] found results from a study of n=522 Type 2 Diabetes patients with DKD were in accordance with published data, showing a deleterious effect of TNFR1 serum concentrations on renal outcomes.
Further evidence continues to mount for how TNFR biomarkers could be used to improve diabetic patient management and outcomes through early intervention. Lopes-Virella et al. [8] have shown in a large cohort of type 1 diabetes patients, followed for six years, how high levels of sTNFR1 and sTNFR2 can predict progression to macroalbuminuria in patients completely free of disease at baseline. TNFR biomarkers can also help doctors to stratify patients with early kidney disease according to the risk of ESRD. Skupien et al [9] show a strong association between a single baseline measurement of TNFR2 serum concentration combined with measurement of HbA1c levels and the future rate of renal function decline in T1DM patients with proteinuria. Identifying patients at highest risk can ensure they are enrolled in therapeutic programmes to delay the rapid decline in renal function.
The future management of kidney disease
Recent statistics show that 25-40% of patients with diabetes are at significant risk of progression to ESRD and cardiovascular morbidity and mortality [10]. The global increase in the incidence in Type 2 diabetes will put more pressure on healthcare systems making it imperative to identify patients at risk of progressive diabetic kidney disease, and initiate protective renal and cardiovascular therapies. Improving outcomes for chronic kidney disease in diabetic patients also has an important impact on mortality; for example, compared with non-diabetic individuals, patients with Type 1 diabetes have no increase in mortality in absence of DKD [11]. There is now solid evidence that sTNFR1 and sTNFR2 can be useful as biomarkers to predict the progression of kidney disease – and not just in patients with diabetes: recent research in Sweden has shown how circulating sTNFRs are relevant biomarkers for kidney damage and dysfunction in elderly individuals in a community setting [12].
Current treatments for CKD, such as control of hypertension and lifestyle interventions (weight loss, diet control, smoking cessation), can reduce the risk of progression to ESRD; therefore, an advanced knowledge of disease risk up to 10 years in advance that the sTNFR1 test kit can provide would be an extremely valuable tool to effectively prevent or reduce morbidity and mortality. Significantly, the sTNFR1 test is also contributing to the development of new targeted therapies aimed at delaying or halting decline in renal function.
References
1. Hasegawa G et al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991; 40: 1007 –1012.
2. Niewczas MA et al. Serum concentrations of markers of TNF alpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2009; 4: 62-70.
3. Ghoda T et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in Type 1 diabetes. J Am Soc Nephrol. 2012; 23: 516-24.
4. Niewczas MA et al. Circulating TNF receptors 1 and 2 predict ESRD in Type 2 Diabetes. J Am Soc Nephrol. 2012; 23: 507-15.
5. Guo G et al. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol. 1999; 277: F766–F772.
6. Forsblom C et al. Added Value of Soluble Tumor Necrosis Factor Alpha Receptor-1 as a Biomarker of ESRD Risk in Patients With Type 1 Diabetes. Diabetes Care 2014; 37: 1–9.
7. Saulnier et al. Association of Serum Concentration of TNFR1 With All-Cause Mortality in Patients With Type 2 Diabetes and Chronic Kidney Disease: Follow-up of the SURDIAGENE Cohort Published online before print March 12, 2014, doi: 10.2337/dc13-2580.
8. Lopes-Virella MF et al. Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 2013; 36: 2317-23. doi: 10.2337/dc12-2521.
9. Skupien et al. Synergism between circulating tumor necrosis factor receptor 2 and HbA1c in determining renal decline during 5-18 years of follow-up in patients with type 1 diabetes and proteinuria. In press: Accepted for publication in Diabetes Care, April 22, 2014.
10. MacIsaac RJ. Markers of and Risk Factors for the Development and Progression of Diabetic Kidney Disease.American Journal of Kidney Diseases 2014; 63: S39–S62.
11. Orchard TJ et al. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010; 53: 2312– 2319.
12. Carlsson AC et al. Soluble TNF Receptors and Kidney Dysfunction in the Elderly. J Am Soc Nephrol. 2014; 25: 1313-1320.
The author
Fergus Fleming
EKF Diagnostic Holdings Plc
Cardiff, UKwww.ekf-diagnostic.com