Trace elements and clinical chemistry
Patients are routinely monitored for levels of trace elements to investigate situations of deficiency or toxicity. This article covers some of the reasons why trace elements are investigated in the clinical setting and discusses, with examples, how the measurements are carried out using advanced analytical instrumentation. It then goes on to suggest some important new developments in the field of inorganic mass spectrometry, which could have an important impact on future clinical assays.
by Dr Chris Harrington
Trace elements are defined as having a concentration of less than 100 µg/g or 100 mg/L and traditionally there are two main reasons for their measurement in a clinical setting: for the determination of deficiency or toxicity.
There are about 10 inorganic micronutrients essential for human health which include the transition elements Cu, Co (as vitamin B12), Cr, Fe, Mn, Mo and Zn, the metalloid Se and the halogen elements F and I. The human body also contains As, B, Ni, Si, Sn and V, but there is no firm evidence any of these are essential for health. These elements have a number of biochemical roles, e.g. co-factors for different enzymes; constituents of important molecules, such as the thyroid hormones; and electron transport, due to their redox chemistry. The toxic effect of any trace element is dose-dependent, but there are a number which exert toxicity at low concentration and examples of these include: Hg, Ti, Pb, Cd and As. The degree of harmful toxicity will not only depend on the concentration, but also on the actual chemical form and exposure time. In the case of an element such as As, it is highly toxic when present as arsine (AsH3) because it is a gas, but when exposure is via a more complex organometallic compound such as arsenobetaine (C5H11AsO2) which is common in fish and seafood, an equivalent dose of As would be harmless. Commonly exposure to a toxic trace element is determined by analysis of a urine sample normalized to the creatinine concentration and comparison to an established guidance value such as a biological exposure index (BEI). However, clearly in the case of As, a measurement of total As in the urine will not differentiate between different chemical forms. To achieve this aim each of the separate As-containing species will need to be determined using methods based on elemental speciation, whereby a chromatographic separation is coupled to a suitable atomic spectroscopic detector, for example HPLC-ICP-MS (inductively coupled plasma mass spectrometry in combination with HPLC).
A more recent development in the clinical measurement of trace elements relates to the orthopedic area and the increasing use of metal alloys containing Cr, Co, Mo and Ti as the components of metal-on-metal (MoM) hip replacements. As a result of complications with the use of such implants and the potential for failure requiring revision surgery, all patients in the UK with MoM replacements are now monitored on an annual basis. The guidelines issued by the UK Medicines and Healthcare products Regulatory Agency (MHRA) in 2010 [MDA/2010/033] and subsequently updated in 2012 [MDA/2012/008 and 036] provide advice to healthcare professionals involved in the management of patients implanted with MoM hip replacements. The initial alert recommended that all patients should be followed up regularly by measurement of Co and Cr in whole blood samples and that this should be carried out most frequently on patients with symptoms consistent with high rates of failure. The medical device alert stipulates that if either element was elevated above a concentration of 7 µg/L (134 and 119 nmol/L for Cr and Co respectively), then further tests should be performed including imaging, to identify patients with potentially failing MoM hip joints. Whereas there are already action limits for these elements relating to occupational exposure, the concentration of 7 µg/L was chosen after consultation with orthopedic clinicians and using information from the National Joint Registry for England and Wales, as a level at which the joint was not showing optimum performance. It was not set as an indication of toxicity but rather as an indicator of joint performance and is thus interpreted with this in mind.
Internal quality control and external quality assurance are important prerequisites for measuring trace elements and making appropriate diagnosis or treatment decisions. A good example of this is the routine annual follow-up of patients with MoM hip-replacements, where clinicians need to make sure that their decisions are based on well controlled analytical measurements. How, for instance, can a clinician decide if an increase in concentration of Co or Cr results from a change in the particular joint and does not arise from a change in the laboratories measurement performance? We recently looked at data [1] from the UK National External Quality Assessment Scheme for trace elements (TEQAS). This supplies whole blood specimens which are spiked with known amounts of a number of trace elements including Co and Cr. The mean recovery over the samples measured in the 2011–12 scheme year was 96.4% (SD 2.23, CV 2.3%) for Co and 96.1% (SD 3.19, CV 3.3%) for Cr. The excellent agreement between the amounts in the specimens and the mean value indicates the results are accurate, and agreement between the pools distributed on different occasions shows they are reproducible over time. This should provide the necessary confidence to the clinical decision maker that the laboratories providing the Co and Cr results are competent and the results are suitably accurate.
Analytical instrumentation
The instrumental mainstay of clinical laboratories which specialise in the measurement of trace elements is inductively coupled plasma mass spectrometry (ICP-MS), which is a form of inorganic MS measuring elemental ions rather than molecular ions. Developed as a commercial analytical technique in the early 1980s it was initially used in environmental and geological laboratories, but after instrumental improvements it is now gaining popularity in the clinical area. This is mainly because it is multi-elemental in nature.
A review of new research and instrumental approaches in the elemental analysis of clinical and biological materials, foods and beverages is published annually as an Atomic Spectrometry Update [2].
The instrumentation itself will not be discussed as many texts [3] deal with the fundamentals of ICP-MS. However, the significant strengths of the technique include: multi-elemental detection in a single run; wide elemental coverage up to m/z 254 (UO+); high sensitivity with low limits of detection, down to sub ng/L levels (limited by purity of the reagents); fast analysis times as a result of the scanning speed of the quadrupole analyser; wide linear working range, up to 9 orders of magnitude in the same run; isotopic information, making high accuracy calibration via isotope dilution mass spectrometry available; and it can be used for specialist applications such as speciation analysis, where it is used as a chromatographic detector for HPLC, GC, CE or GE separations. The main weaknesses of the technique are: the presence of isobaric interferences on some elements, which mean the isotope of one element is at the same m/z ratio for the analyte of interest, for instance Ca has an isotope at m/z 48 which is the same as the most abundant isotope for Ti, making the measurement of Ti in clinical samples problematic; the formation of polyatomic ions from sample matrix and atmospheric ions can impinge on the m/z for the analyte of interest, an example would be the measurement of Cr at its most abundant isotope at m/z 52 which has a major interference from the formation of ArC; and the formation of doubly charged ions, for instance Gd2+ can interfere with Se at m/z 78. Luckily instrumental developments based on the use of a reaction/collision cells containing a suitable gas have been introduced to overcome the problems due to polyatomics and doubly charged ions. These work by using a reactive gas, e.g. H2 in the cell and removing the interference by reactively neutralising it, which we have recently demonstrated for the removal of the Gd2+ interference from the measurement of Se [4], or using a collision gas, e.g. He to remove the larger polyatomic ions by collision induced kinetic energy discrimination. These newer instruments are extremely robust and can rapidly deliver highly accurate measurements for multi-elements at low concentrations in difficult matrices such as whole blood, serum or urine.
Future trends and developments
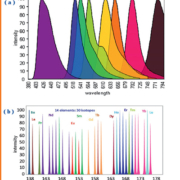
Over the last 5–10 years the capabilities of ICP-MS for the detection of molecules that do not contain a trace element have been investigated. By using a reagent with specificity for the analyte and which carries a metal or nanoparticle tag, the molecule of interest becomes visible for detection by ICP-MS. The reagents used are often antibodies, so the protocols often mimic those developed for immunochemical assays and, in theory, can be applied to the determination of the same analytes, including peptides, proteins and other specific biomarkers. So why would this be advantageous compared to conventional immunochemical assays? Most importantly this approach has a greater potential for multiplexing than spectroscopic methods; there are a large number of elemental tags to choose from and no overlap between them. As illustrated in Figure 1 this is not the case with fluorescence signals.
Other advantages include: analyte quantification with high precision; low detection limits; large dynamic range; low matrix effects from other components of the biological sample (i.e. contaminating proteins in the sample have no effect on elemental analysis); low background from plastic plates (i.e. plastic containers do not cause interference on elemental detection as it can with fluorescence), and superior spectral resolution. As can be seen in Figure 1 this has generated a new approach to flow-cytometry based on ICP-MS and it’s very likely that other new approaches to other biochemical analytes will shortly become commercially available.
Acknowledgements
Scott Tanner, University of Toronto for permission to use the figures from the CyTOF flow cytometer based on ICP-ToF-MS (DVS Sciences Inc).
References
1. Harrington CF, Taylor A. BMJ 2012; 344: e4017.
2. Taylor A, Day MP, Hill S, Marshall J, Patriarca M, White M. J Anal At Spectrom. 2013; 28: 425–459.
3. Inductively Coupled Plasma Mass Spectrometry Handbook. Ed. Nelms S, Blackwell 2005.
4. Harrington CF, Walter A, Nelms S, Taylor A. Ann Clin Biochem. 2013; submitted.
The author
Chris Harrington PhD, MRSC
SAS Trace Element Laboratory, Faculty of Health and Science, University of Surrey, Guildford, Surrey, GU2 7XH, UK
E-mail: Chris.harrington1@nhs.net