TSHR mRNA: a peripheral blood marker to diagnose differentiated thyroid cancer
Thyroid cancer is the most common endocrine malignancy and the fastest-growing cancer in terms of incidence rates to affect women. In the last decade, thyroid cancer rates have increased in most populations worldwide. Enhanced diagnostic tools for thyroid cancer have become available and stand to impact decision-making in patient care, the extent of surgical treatment, and prognosis in long-term follow-up. This article discusses how the novel blood test TSHR mRNA plays a role in these clinical scenarios.
by Dr Mira Milas and Dr Manjula Gupta
Differentiated thyroid cancer is represented most frequently by papillary thyroid cancer and follicular thyroid cancer, with other histological variants in the mix. These cancers originate from the follicular cells which comprise the thyroid gland and produce the protein thyroglobulin (Tg) and also the essential hormones thyroxine (T4) and triiodothyronine (T3). Unlike medullary thyroid cancer, where calcitonin elevation measured from a simple blood test is virtually diagnostic of the disease, papillary and follicular cancers have no such marker. Tg functions only to detect thyroid cancer recurrence in the absence of the thyroid gland, not pre-operatively. Thus, the mainstay of initial diagnosis of the differentiated thyroid cancers has been cytology, obtained by fine needle aspiration biopsy (FNAB) of thyroid nodules that are detected by exam or by ultrasound imaging [1].
FNAB would be an excellent diagnostic tool if it allowed reliable and consistent diagnosis of differentiated thyroid cancers, but it has several limitations. Its sensitivity and specificity overall are 95% and 48%, and the positive and negative predictive values are 68% and 89%, respectively [2]. When the aspirate from a thyroid nodule contains all the morphologic features of papillary thyroid cancer, FNAB is 99% accurate in designating this malignancy. However, up to 40% of aspirates are abnormal but cannot be classified into a malignant subtype. Follicular thyroid cancer and the follicular variant of papillary thyroid cancer are examples of this – there are simply no morphological changes specific enough to allow recognition of such cancers via microscopic examination of biopsy samples. Additional complexity comes from the considerable variability among interpretations of thyroid cytology samples, both among different pathologists and when the same pathologist views the same sample at different times [2]. In 2008, a new classification system – the Bethesda system for reporting thyroid cytopathology – was developed to address this variability [3].
For those patients with thyroid nodules whose FNAB results fall into an abnormal category without definitive evidence of malignancy (Bethesda categories III, IV, V), the traditional management algorithm has relied on surgery to enable diagnosis. Although thyroidectomy in expert hands has low complication rates, these are still measurable, and most notable for the 1-3% risk of permanent voice hoarseness. Most thyroid specialists have long appreciated that using thyroid surgery in this diagnostic capacity is not ideal, especially when 60-80% of patients will be found to have benign thyroid histology.
In these patients, surgery could have been potentially avoided altogether if better diagnostic markers existed. In other patients, who undergo only partial thyroidectomy initially, a second surgery becomes necessary when thyroid cancer is confirmed. This group would benefit from markers that reliably indicated thyroid cancer at the outset, so that the appropriate extent of thyroid surgery could be accomplished at the first operation.
It is no wonder that these suboptimal clinical scenarios inspired decades of investigation into potential molecular markers of thyroid cancer [4]. Several promising innovations have come to direct availability for patient care in the last few years, and they represent very different strategies. Some investigators focused on detecting known gene mutations (e.g. BRAF, RAS, PTC/RET) associated with thyroid cancer from the aspirated specimens in FNAB. This was pioneered by the work of Nikoforov and colleagues and acknowledged as a potential diagnostic tool in the American Thyroid Association 2009 guidelines for management of thyroid nodules and thyroid cancer [1,5]. In 2011, testing for these thyroid cancer-related mutations in FNAB became commercially available. Another group of investigators also focused on information obtained via FNAB samples, but developed a 142-gene profile that would classify the nodule as benign, potentially allowing surgery to be avoided or postponed. This effort was based on a multi-institutional study and acquisition of large sample cohorts, also leading to a commercially available product in 2011 [7, 8 and see this issue of CLi, page 14].
In contrast to such tissue-based strategies, the TSHR mRNA molecular marker is derived from a peripheral blood test sample and was first available for routine testing in October 2008. It functions as a surrogate marker for circulating thyroid cancer cells, and is not detectable in individuals who have normal thyroid glands. As a blood test, it is convenient to obtain and can be measured at various time intervals, thus functioning both for initial diagnostic purposes and for later roles in prognosticating outcome from thyroid cancer surgery.
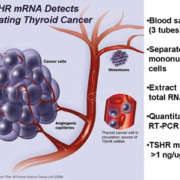
In 2002, investigators at the Cleveland Clinic, USA, led by Dr Manjula Gupta first reported the method of detecting TSHR mRNA from peripheral blood samples of patients [9], [Figure 1]. It relied on the separation of mononuclear cells from the buffy coat layer, total RNA extraction and then RT-PCR using a patented primer pair that was found to be most effective in pre-clinical studies. In 2007, the assay technique switched to quantitative RT-PCR, defining a threshold level of TSHR mRNA >1 ng/ug total RNA to indicate the presence of thyroid cancer [10]. This technique was estimated to have a sensitivity that detects fewer than ten thyroid cancer cells per one mL of blood.
Studies that were conducted at the Cleveland Clinic since 2002 elucidated the following important aspects of TSHR mRNA [summarised in reference 11].
- It came to represent the first consistent use of a circulating molecular marker for pre-operative diagnosis of thyroid cancer and for post-operative thyroid cancer status assessment.
- Quantitative levels of TSHR mRNA differed among various thyroid disease states and subtypes of thyroid cancer. Notably, TSHR mRNA levels distinguished between benign goitres and thyroid cancer, especially recurrent thyroid cancer.
- Thyroid microcarcinomas (<1 cm size) had a detection rate by TSHR mRNA comparable to tumours >1 cm, implying that cancer cells are shed into the circulation even at an early stage.
- For indeterminate FNAB samples in the follicular neoplasm category, elevated TSHR mRNA levels by themselves had a 96% positive predictive value for thyroid cancer. In an algorithm that combines TSHR mRNA and ultrasound features of the thyroid nodule, 100% of cancer patients are steered correctly to initial total thyroidectomy while a third of patients with benign disease avoid surgery.
- In patients with elevated pre-operative levels, TSHR mRNA disappears on day 1 after thyroidectomy. Persistently elevated levels correlated with residual thyroid cancer or early recurrence.
- TSHR mRNA showed promise in long-term thyroid cancer follow-up, especially in individuals in whom the traditional monitoring of Tg levels is unreliable because of antibodies against Tg.
The utility of TSHR mRNA in the management of patients with thyroid disease was validated in an additional large cohort of patients following the availability of TSHR mRNA for routine use [11]. The patterns of marker use in this study confirmed that clinicians – other than the investigators involved with the marker development – found TSHR mRNA helpful in their daily decision-making. The scenarios below are selected to highlight some of the more pertinent clinical applications of TSHR mRNA.
TSHR mRNA as an initial diagnostic marker for differentiated thyroid cancer
A thought-provoking way in which to view TSHR mRNA is that, as a solo diagnostic tool, its overall performance resembles in some ways that of thyroid nodule FNA. Original ROC curve analysis of TSHR mRNA performance identified a sensitivity of 72%, specificity of 83% and AUC as 0.82. The positive and negative predictive values were 81% and 64%, respectively. These values remained constant for the validation cohort, and slightly improved to the mid-80% range in all parameters in recent clinical verification [2012, unpublished laboratory quality control data]. Thus, it is interesting to consider whether, in patients with thyroid nodular disease, measurement of TSHR mRNA could serve as a first line of investigation, potentially avoiding the more painful and costly FNAB, or channeling use of FNAB to more selected individuals.
At present, TSHR mRNA is always used in conjunction with existing clinical standards of care. The most useful application of TSHR mRNA is in clarifying the diagnosis of follicular neoplasms –Bethesda category IV thyroid lesions – to facilitate initial diagnosis of thyroid cancer. As used at the Cleveland Clinic, TSHR mRNA is measured in all patients who have this FNAB cytology result, at least one week following the biopsy or at the time of routine pre-operative laboratory testing. Patients with an elevated TSHR mRNA level would be advised to have total thyroidectomy at the initial surgery based on the high risk of thyroid cancer (96%). As with any important medical decision, a thoughtful discussion of risks, benefits and alternative approaches takes place with the patient, taking into account the entire medical history that may be relevant to an individual. Thus, patients may still elect to proceed with a stepwise process of diagnostic hemithyroidectomy (lobectomy), followed by a second surgery for complete removal of the thyroid gland, if that meets their own expectations better or is a more acceptable treatment. Similarly, patients whose TSHR mRNA levels are undetectable and whose thyroid nodule has benign features may be candidates for observation, following a similar informed consent process. This algorithm and its success in the most recent validation cohort are summarised in Figure 2.
TSHR mRNA can also be combined with other molecularly-based strategies for thyroid cancer detection in nodules. This is a synergistic and desirable approach because, despite outstanding progress, none of the current diagnostic tools (FNAB or molecular markers) are perfect. Viewed quite realistically, their independent performance characteristics are modest to pretty good, but they represent the best tools currently available. It is important to become knowledgeable about the proper application and usage of the diagnostic tools, their expected outcomes and whether the data are sufficiently compelling to sway clinical decision-making. Consider, for example, a patient whose thyroid FNAB sample had no detectable gene mutations. This scenario actually occurs most often. Estimates of undetected mutations were reported in 90% of atypical (Bethesda III) thyroid samples and 82% of follicular neoplasms, reflecting the genetic heterogeneity of thyroid cancers [12]. The thyroid nodule may nevertheless harbour malignancy, and potentially this could be picked up by the TSHR mRNA measurement in peripheral blood. Alternately, the Afirma gene profile sorts thyroid nodules into the benign category, but does not provide risk stratification for cancer if the answer is ‘not benign’ [7, 8]. Here, TSHR mRNA measurement could gauge the likelihood of cancer and thus, again, potentially allow a surgeon to perform an appropriate extent of thyroid surgery at the 1st operation.
TSHR mRNA in the evaluation of multinodular goitre
Because it is a blood test, TSHR mRNA has versatility that, by definition, is unavailable from tissue-based markers. Thus, we observed that endocrinologists, particularly, were eager to measure TSHR mRNA levels in patients with multinodular goitres in the following scenarios: presence of too many nodules to effectively biopsy, reluctance of the patient to undergo FNAB, enlargement of thyroid nodules despite benign FNAB, the presence of mild criteria to undergo thyroid surgery for goitre with a desire (on the part of the patient, physician or both) to have more convincing reasons (such as cancer risk) that surgery is necessary. Figure 3 summarises the findings in such patients, demonstrating that elevated TSHR mRNA may facilitate identification of patients whose goitre disease may benefit from consultation with a surgeon, if not surgery itself.
TSHR mRNA in long-term prognosis and follow-up of thyroid cancer
A remarkable finding from our clinical use of TSHR mRNA was that it disappears from circulation as early as 24 hours after total thyroidectomy. In patients who were confirmed to have thyroid cancer, persistent elevation of TSHR mRNA at this timepoint occurred in 15% of cases. These individuals manifested persistent local disease, recurrence in cervical lymph node, lung or bone metastases, or histologic features of aggressive disease (tall cell subtypes, angiolymphatic invasion, extrathyroidal extension). We remain interested in the utility of TSHR mRNA for long-term prognosis of thyroid cancer. We also continue to follow thyroid cancer patients prospectively with TSHR mRNA, adding it to the panel of other modalities most commonly used for this purpose (radioiodine whole body scan, neck ultrasound, Tg and TgAB monitoring, physical exam). Those individuals whose TSHR mRNA levels suggest persistent or recurrent thyroid cancer not apparent by other means are monitored more closely [11,13].
TSHR mRNA has demonstrated a consistent performance as a unique marker in the field of thyroid diseases. Thus far, it remains the only available blood test that is molecularly-based and that can predict risk of differentiated thyroid cancer before surgery or needle biopsy. Ideally, its use can be envisioned as a key component in a multi-modality panel of options that are knowledgeably applied to appropriate clinical scenarios. Ultimately, TSHR mRNA is an innovative tool for the outlook of the modern time – it allows for a personalised diagnostic and treatment approach for each individual with thyroid nodular disease and thyroid cancer.
References
1. Cooper DS et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009 Nov;19(11):1167-214.
2. Wang CC et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011 Mar;21(3):243-51.
3. The Bethesda System for Reporting Thyroid Cytopathology. Ali SZ and Cibas ES, editors. Springer 2010.
4. Barbosa FG, Milas M. Peripheral thyrotropin receptor mRNA as a novel marker for differentiated thyroid cancer diagnosis and surveillance. Expert Rev Anticancer Ther 2008;8(9):1415-1424
5. Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid 2009 Dec;19(12):1351-61.
6. http://www.asuragen.com/ClinicalLab/informthyroid/informthyroid.aspx
7. Chudova D et al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J Clin Endocrinol Metab 2010 Dec;95(12):5296-304.
8. http://www.veracyte.com/
9. Gupta MK et al. Detection of circulating thyroid cancer cells by reverse transcription-PCR for thyroid-stimulating hormone receptor and thyroglobulin: the importance of primer selection. Clin Chem 2002;48:1862-5.
10. Chia SY et al. Thyroid-stimulating hormone receptor messenger ribonucleic acid measurement in blood as a marker for circulating thyroid cancer cells and its role in the preoperative diagnosis of thyroid cancer. J Clin Endocrinol Metab 2007;92:468-475
11. Milas M et al. Circulating Thyrotropin Receptor (TSHR) mRNA as a Novel Marker of Thyroid Cancer: Clinical Applications Learned from 1,758 Samples. Annals of Surgery 2010; 252(4):643-51,
12. Nikiforov YE et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab 2011 Nov;96(11):3390-7.
13. Milas M et al. Effectiveness of peripheral thyrotropin receptor mRNA in follow-up of differentiated thyroid cancer. Ann Surg Oncol 2009 Feb;16(2):473-80.
The authors
Mira Milas, MD, FACS and Manjula Gupta, PhD
Cleveland Clinic Lerner College of Medicine
Department of Endocrine Surgery
Endocrinology and Metabolism Institute
9500 Euclid Avenue F20
Cleveland, OH 44195, USA
e-mail: milasm@ccf.org