Tumour markers for oropharyngeal cancers
Head and neck cancers (HNC) are a globally prevalent malignancy. Despite the efforts of reducing several known etiological factors such as smoking and drinking to lower the incidence of HNC at the population level, the incidence of oropharyngeal cancers (OPC) is on the rise. OPC is caused by human papillomavirus (HPV) and most prevalent in a younger age group. This review critically examines the epidemiology, biology and laboratory detection of OPC and provides future insights into combating this debilitating disease.
by X. C. Sun, P. Tran and Dr C. Punyadeera
Introduction
Head and neck cancers (HNC) are the sixth most prevalent neoplasm in the world with approximately 650 000 cases diagnosed each year [1–5]. Oral and oropharyngeal squamous cell carcinomas (OSCC & OPSCC) together constitute 90% of malignancies in the head and neck region. Several known traditional etiological factors such as tobacco and alcohol use are recognized in the development of these cancers. More recently, human papillomavirus (HPV) infection is recognized as an additional risk factor for the development of a subset of HNCs, mainly OPSCC [6].
In recent decades, the overall incidence of HNC caused by smoking and alcohol is on the decline. In contrast, HPV+ve OPSCC is on the rise. In developed countries such as the United States of America, the incidence of HPV+ve OPSCC is escalating, with predictions that more than 50% of patients will be HPV+ve by 2030 [7]. Interestingly, patients who are HPV+ve OPSCC are relatively younger than HPV-ve HNC patients and are therefore less likely to have any history of chronic or excessive alcohol or tobacco use but are more likely to engage in social habits that increase the likelihood of HPV transmission (oral sex). The clear distinction between HPV+ve OPSCC and HPV-ve cases provides multiple downstream inputs that can be applied into clinical treatment modalities. Conversely, it provides an exciting opportunity for the development of early diagnostic and screening methods to combat HNC at a population level through prevention strategies.
HPV+ve OPSCC are both clinically and biologically distinct tumour entities compared with HPV-ve counterparts. Classically, HPV+ve OPSCC patients present with a molecular profile that includes retinoblastoma (pRB) pathway inactivation, p53 degradation and p16 upregulation. Clinically, HPV+ve OPSCC patients often present with smaller primary tumours but more advanced nodal disease, similar rates of metastasis and differing patterns of metastasis [8, 9]. In addition, patients with HPV+ve tumours have better prognosis with 5-year survival at 75% (c.f. 25% for HPV-ve patients). There are a number of techniques for the diagnosis and detection of HPV+ve OPSCC, including histopathology, polymerase chain reaction (PCR) and immunohistochemistry (IHC).
Biology
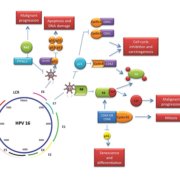
Upon integration of HPV DNA into the host genome, E6 and E7 viral oncoproteins activate a number of pathways within the host cell. The primary molecular target of E7 is the Rb protein and the E7 viral oncoprotein reprogrammes terminally differentiated epithelial cells to re-enter the cell cycle. E7 disrupts the Rb–E2F complex leading to the release of E2F, subsequently resulting in cyclin A and E activation and entry of the cell into S phase. As a consequence p16 is overexpressed [10, 11]. The E6-associated protein (E6-AP) is a specific ubiquitin-ligase that binds to the viral E6 oncoprotein, resulting in p53 degradation. E6 and E7 have also been shown to interfere with growth inhibitory cytokines [such as tumour necrosis factor-α (TNFα)] and to disrupt the mitochondrial apoptotic pathway by interfering with pro-apoptotic BAK and BAX [10]. E6 and E7 alone are insufficient to cause malignant cell transformation; however, due to their interference with proliferation checkpoints and apoptotic pathways, it is certain that the accumulation and damaged DNA, mitotic defects and integration of foreign DNA substantially increase the risk of malignant progression [10].
Detection methods
A number of diagnostic methods are currently available to evaluate whether a tumour is HPV+ve. These methods include both indirect as well as the direct methods; i.e. the latter includes the detection of HPV genomic DNA (gDNA). Besides clinical examination, current methods for the diagnosis of HPV status include tissue biopsy staining for p16 (indirect method). Biopsies may fail when tumours are too small to access or when they are located in hidden anatomical sites [10]. Other methods include the detection of HPV gDNA using PCR and in situ hybridization (ISH) as well as the detection of HPV viral transcripts E6 and E7 by PCR.
p16 detection by IHC is widely used in cervical cancer cases for the detection of HPV and it is being studied extensively in the field of HNC [12]. During immortalization of host cells, the E7 protein binds to Rb, resulting in the compensatory overexpression of the tumour suppressor gene p16 in HPV-infected tumour cells. Therefore, IHC detection of p16 is considered as an indirect surrogate marker to determine the presence of HPV [11]. However, there are pitfalls associated with p16 IHC detection. A number of studies have shown suboptimal specificity of IHC [10, 11]. As a consequence of the extreme anatomical and biological heterogeneity in HNC, elevation of p16 by non-viral materials may contribute to a considerable false positive rate [11]. Although it has been reported that p16-positive patients have a better prognosis and increased radiosensitivity, it has been advised that p16 detection by IHC alone cannot accurately identify HPV infection in HNC [12].
Detection of HPV gDNA is a widely used method because of its high sensitivity and cost-effectiveness. Common primers (MY09/MY11 and GP5/GP6) that target the L1 open reading frame are used to detect wide-spectrum HPV genotypes [11]. However, standard PCR primers do not allow detection of specific HPV genotypes [10]. In addition, the target L1 region could also be deleted upon viral integration, which may affect sensitivity of the test [10, 11]. Although, specific E6 and E7 primers have been designed and used to overcome L1 deletion, this method still lacks the ability to distinguish stromal/tumour and episomal/integrated DNA materials and is prone to contamination interference, which undermines the clinical usefulness [11].
The HPV DNA ISH method is unique because of its high specificity and the ability to be evaluated microscopically. The visible hybridization signals that precipitate within the nuclei help distinguish integrated and episomal DNA [11]. It is noteworthy that the presence of HPV DNA detected by ISH significantly correlates with p16 detection by IHC. [10]. However, ISH methods carry lower sensitivity compared to its excellent specificity [11]. The detection of HPV-16 viral transcripts E6 and E7 can highlight whether a patient is suffering from persistent infection – information that is clinically more valuable [12]. However, because of the fragile nature of mRNA, formalin-fixed paraffin-embedded (FFPE) specimens are often not ideal for RNA analysis and frozen fresh specimen are required [12].
The detection of HPV-specific IgG in serum is a useful biomarker to determine previous and current HPV infection status [13]. Serological biomarkers are not site-specific, and can arise due to HPV infections at sites other than the oral cavity, hence potentially affecting the specificity of the assay.
The effect of Gardasil™
From treatment and management of HPV-related diseases, the paradigm of HPV care has shifted to a preventative approach since the breakthrough introduction of the HPV vaccine, Gardasil™ (Merck & Co.). The biologic basis of HPV vaccines relies on the mechanism of neutralizing antibodies generated against virus-like particles (VLP), which consist of the major capsid protein HPV L1 [10]. The quadrivalent HPV 6/11/16/18 vaccine Gardasil™ was licensed by the FDA to prevent cervical, vaginal and vulvar infections in women in 2006 and genital warts in men in 2009, followed by the licensing of bivalent HPV 16/18 vaccine Cervarix™ (GlaxoSmithKline), in women in 2009 [14].
The benefits of HPV vaccination for the oral cavity include not only the biologically-plausible direct effect on oral infections, but also the sequential oral infection reduction following genital infection reduction, due to the sexually transmitted nature of HPV. To date, there are only a few studies examining the effect of Gardasil on oral infection; however, the results showed a promising outlook with high vaccine efficacy (as high as 93% in 4 years time, as recorded by randomized controlled trial in Costa Rica) [15] and reduced viral prevalence (oral prevalence dropped to 1.4% from 9.3% in the 15–23 age group in youth clinics in Sweden) [16].
Future outlook
As previously mentioned, HPV-related OPSCC involve a new segment of the population, which is distinctively different to the traditional HNC patient cohort caused by excessive smoking and drinking. This requires clinicians to conduct thorough cancer screening of at-risk groups. Such screening programmes should pay particular attention to cervical lymph nodes as some subtypes of HNC, especially OPSCC, involve hard-to-examine areas for clinical visual examination.
As a result of the sexually transmitted nature of HPV, some studies have advocated routine sexual behaviour education in clinical practice. However, this practice carries inherent controversies of sexual harassment and confidentiality [17]. Public awareness campaigns have been argued to be a more efficient preventative means in altering patients’ behaviour. Studies have shown that many oral health practitioners have limited knowledge with regards to HPV-related HNC and HPV vaccinations [17]. Professional bodies and health authorities are required to address this knowledge gap by establishing new clinical guidelines and using continuing educational methods, in order to effectively control the rising trend of HPV-related HNC.
Conclusion
All current diagnostic methods require excision of tumour tissue and this can be challenging when they are located in hidden sites. Efforts have been made globally to develop a less invasive, more cost-effective and clinically-relevant test. Serology tests that detect HPV-specific IgG have been shown to indicate viral presence and are linked with prognostication; however, this method inherently lacks site specificity [10]. Oral specimens, more specifically oral rinse, have shown promise in this field. Oral rinse samples not only are non-invasive and cost-effective, the proximity of collection to the area of interest ensures the localized sampling field. It is also important to note that shedding of normal cells into the oral cavity/oral pharynx may interfere with and/or decrease the HPV detection level [10]. OraRisk® HPV test, uses oral rinses for HPV detection [10]. Translational collaborations between scientists and clinicians have resulted in an assortment of tumour markers and diagnostic techniques for OPSCC. However, these need to be tested in clinical trials to determine the cost-effectiveness.
Acknowledgments
This work is supported by Garnett Passé & Rodney Williams Memorial Foundation and the Queensland Centre for Head and Neck Cancer funded by Atlantic Philanthropies, the Queensland Government and the Princess Alexandra Hospital.
References
1. Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011; 57(5): 675–687.
2. Nagadia R, Pandit P, Coman WB, Cooper-White J, Punyadeera C. miRNAs in head and neck cancer revisited. Cell Oncol (Dordr). 2013; 36(1): 1–7.
3. Ovchinnikov DA, Cooper MA, Pandit P, Coman WB, Cooper-White JJ, Keith P, Wolvetang EJ, Slowey PD, Punyadeera C. Tumor-suppressor gene promoter hypermethylation in saliva of head and neck cancer patients. Transl Oncol. 2012; 5(5): 321–326.
4. Kulasinghe A, Perry C, Jovanovic L, Nelson C, Punyadeera C. Circulating tumour cells in metastatic head and neck cancers. Int J Cancer 2015; 136(11): 2515–2523.
5. Ovchinnikov DA, Wan Y, Coman WB, Pandit P, Cooper-White JJ, Herman JG, Punyadeera C. DNA methylation at the novel CpG sites in the promoter of MED15/PCQAP gene as a biomarker for head and neck cancers. Biomark Insights 2014; 9: 53–60.
6. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009; 45(4–5): 309–316.
7. Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012; 6(Suppl 1): S16–24.
8. Galbraith NS. Infectious disease control. BMJ 1990; 300(6738): 1477–1478.
9. Powell NG, Evans M. Human papillomavirus-associated head and neck cancer: oncogenic mechanisms, epidemiology and clinical behaviour. Diagn Histopathol. 2015; 21(2): 49–64.
10. Chai RC, Lambie D, Verma M, Punyadeera C. Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med. 2015; doi: 10.1002/cam4.424.
11. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck 2012; 34(4): 459–461.
12. Bussu F, et al. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br J Cancer 2013; 108(5): 1157–1162.
13. Castle PE, Shields T, Kirnbauer R, Manos MM, Burk RD, Glass AG, Scott DR, Sherman ME, Schiffman M. Sexual behavior, human papillomavirus type 16 (HPV 16) infection, and HPV 16 seropositivity. Sex Transm Dis. 2002; 29(3): 182–187.
14. Sanders AE, Slade GD, Patton LL. National prevalence of oral HPV infection and related risk factors in the U.S. adult population. Oral Dis. 2012; 18(5): 430–441.
15. Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC, Solomon D, Jimenez S, Schiller JT, Lowy DR, van Doorn LJ, Wacholder S, Kreimer AR; CVT Vaccine Group. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 2013; 8(7): e68329.
16. Grün N, Ährlund-Richter A, Franzén J, Mirzaie L, Marions L, Ramqvist T, Dalianis T. Oral human papillomavirus (HPV) prevalence in youth and cervical HPV prevalence in women attending a youth clinic in Sweden, a follow up-study 2013-2014 after gradual introduction of public HPV vaccination. Infect Dis (Lond). 2015; 47(1): 57–61.
17. Daley E, DeBate R, Dodd V, Dyer K, Fuhrmann H, Helmy H, Smith SA. Exploring awareness, attitudes, and perceived role among oral health providers regarding HPV-related oral cancers. J Public Health Dent. 2011; 71(2): 136–142.
18. Salazar C, Calvopiña D, Punyadeera C. miRNAs in human papilloma virus associated oral and oropharyngeal squamous cell carcinomas. Expert Rev Mol Diagn. 2014; 14(8): 1033–1040.
The authors
Xiaohang Charles Sun1, Peter Tran1 and Chamindie Punyadeera*2 MSc, PhD
1School of Dentistry, The University of Queensland, Brisbane, Australia
2The Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Australia.
*Corresponding author
E-mail: chamindie.punyadeera@qut.edu.au