Tumour markers for the diagnosis of mucinous ovarian cancer
The aim of this study was to determine the accuracy of CEA, CA 15.3, CA 19.9 and CA 125 for diagnosis of mucinous ovarian cancer (MOC). We studied 94 women with mucinous ovarian tumour, 82 were NOT MOC (68 mucinous ovarian cystadenomas and 14 mucinous borderline ovarian tumour) and 12 were MOC. All MOC patients were in FIGO stage I or II. No statistically significant differences were found between MOC and NOT MOC patients according to CEA and CA 15.3 (P>0.05). AUC values were 0.862 (P=0.0002) and 0.829 (P=0.0021) for CA 19.9 and CA 125 respectively. In conclusion, preoperative CA 19.9 and CA 125 levels showed high diagnosis efficacy to predict whether a mucinous ovarian tumour is benign or malignant.
by Dr J. D. Santotoribio, A. Garcia-de la Torre, C. Cañavate-Solano, F. Arce-Matute, M. J. Sanchez-del Pino and S. Perez-Ramos
Introduction
Ovarian cancer is the fifth leading cause of cancer-related death in women in developed countries and has one of the highest ratios of incidence to death [1]. Epithelial ovarian cancer is a heterogeneous disease with a heterogeneous distribution pattern [2]. Epithelial ovarian cancer set by the World Health Organization recognizes eight histological tumour subtypes: serous, mucinous, endometrioid, clear cell, transitional cell, squamous cell, mixed epithelial and undifferentiated [3]. Mucinous ovarian cancer (MOC) is an epithelial ovarian cancer that contains cysts and glands lined by mucin-rich cells and historically accounted for approximately 11.6% of all primary epithelial ovarian carcinomas [4]. MOC should be considered separate from the other epithelial ovarian cancers as metastatic primary disease and recurrent mucinous cancers have a substantially worse prognosis than other epithelial ovarian cancers [5]. Tumour markers are biochemical substances found in the blood which may be measured for the diagnosis of cancer. The major challenge of developing a screening test using serum tumour markers, is that it must be highly specific (because of the low prevalence of ovarian cancer) in order to avoid detection of numerous false positives [6]. The most common tumour markers in clinical chemistry are carcinoembryonic antigen (CEA), cancer antigen 15.3 (CA 15.3), cancer antigen 19.9 (CA 19.9) and cancer antigen 125 (CA 125). CEA and CA 15.3 have been found at elevated levels in patients with epithelial ovarian cancer [7–9]. Preoperative elevated CA 19.9 levels are related to a higher probability of MOC [8, 10]. A diagnostic approach based on the use of CA 125 has been suggested for the early diagnosis of ovarian cancer, although premenopausal women may have higher serum CA 125 levels than in postmenopausal women [11–13]. Also, in mucinous borderline ovarian tumours have found a significant relation with elevated CA 125 [14, 15]. Another tumour marker for diagnosis of ovarian cancer, serum human epididymis protein 4 (HE4), has lowest concentrations in mucinous tumours and displays no difference in serum concentration between benign or malignant mucinous ovarian tumours [12, 13].
The aim of this study was to determine the accuracy of CEA, CA 15.3, CA 19.9 and CA 125 for diagnosis of MOC in patients with mucinous ovarian tumors.
Materials and methods
Women with mucinous ovarian tumours diagnosed between 2004 and 2012 were included in the study. We excluded patients with other tumours that could elevate the tumour markers. Before biopsy and after obtaining an informed consent, blood specimens were drawn by venipuncture in gel separator serum tubes and centrifuged at 4000 rpm for 4 min. The following variables were analysed: CEA, CA 15.3, CA 19.9 and CA 125. We measured the serum concentrations of the tumour markers by electrochemiluminescence immunoassay (ECLIA) in MODULAR E-170 (ROCHE DIAGNOSTIC®). The reference range values provided by our laboratory are: CEA (0–3.4 ng/mL), CA 15.3 (0–30 U/mL), CA 19.9 (0–37 U/mL) and CA 125 (0–35 U/mL). After surgery, histology and stage were determined according to the International Federation of Gynecologists and Obstetricians (FIGO) classification. Patients were classified into two groups according to the diagnosis of ovarian biopsy: NOT MOC (mucinous ovarian cystadenomas and mucinous ovarian borderline tumour) and MOC. For all statistical comparisons a value of P<0.05 was considered significant. The accuracy of serum tumour markers was determined using receiver operating characteristic (ROC) techniques by analysing the area under the ROC curve (AUC). The optimal cut-off value was considered with higher than 95% specificity. Statistical analysis was performed using the software MEDCALC®.
Results
We enrolled 94 women aged between 15 and 80 years old (median age was 43). Eighty-two patients (87.2 %) were NOT MOC (68 mucinous ovarian cystadenomas and 14 mucinous ovarian borderline tumours) and 12 patients (12.8 %) were MOC. Thirty-two patients were postmenopausal and 62 patients were premenopausal. All MOC patients were in FIGO I or II stages.
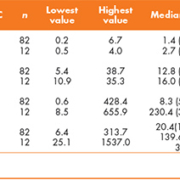
Descriptive statistics of serum tumour markers in MOC and NOT MOC patients are shown in Table 1. No statistically significant differences were found between MOC and NOT MOC patients according to CEA and CA 15.3 (P>0.05). The frequency of abnormal serum levels CA 19.9 and CA 125 in MOC and NOT MOC patients are shown in Table 2. AUC, optimal cut-off value, sensitivity and specificity of ROC curves for diagnosis of MOC using CA 19.9 and CA 125 are displayed in Table 3.
No statistically significant differences were found between premenopausal and postmenopausal women for CEA, CA 15.3, CA 19.9 and CA 125. Also, these tumour markers were not statistically significant for the diagnosis of mucinous borderline ovarian tumours (P>0.05).
Discussion
In the literature, CEA has been noted to be elevated in almost one third of all ovarian carcinomas. CEA is much more likely to be elevated in mucinous ovarian carcinomas than in non-mucinous ovarian carcinomas [5, 7, 8]. CA 15.3 has been found to be elevated levels in patients with advanced epithelial ovarian cancer [8, 9]. However, in this study, CEA and CA 15.3 were not useful to differentiate benign from malignant mucinous ovarian tumours.
In the recent paper of the guidelines on the recognition and initial management of ovarian cancer from the National Institute for Health and Clinical Excellence (NICE) stated that general practitioners should measure serum CA 125 in primary care in women with symptoms that suggest ovarian cancer [11]. Also, a diagnostic approach based on the use of CA 125 in association with ultrasonography has been suggested for the early diagnosis of ovarian cancer [11, 12]. The major drawback of using CA 125 as a screening strategy is that up to 20% of ovarian cancers do not express the antigen, and also that abnormal serum levels CA 125 may be found in patients with benign ovarian tumours [12, 13]. Recently, another tumour marker for ovarian cancer has been proposed, serum human epididymis protein 4 (HE4), frequently overexpressed in ovarian cancers, especially in serous and endometrioid histology [6, 12, 13]. However, HE4 has lowest concentrations in mucinous tumours and shows no difference in serum concentrations between benign or malignant mucinous ovarian tumours [12, 13]. Serum CA 19.9 presents low efficiency for the diagnosis of serous ovarian cancer, but preoperative elevated CA 19.9 levels could be related to a higher probability of MOC [8, 10]. In this paper, CA 125 false positive results (abnormal serum levels) were found in 31.7 % of NOT MOC patients and false negative (normal serum levels) in 33.3 % of MOC patients. CA 19.9 false positive results were found in 19.5 % of NOT MOC group and false negative in 16.6 % of MOC group. All MOC patients had abnormal serum CA 19.9 and/or CA 125 levels, and 60.98 % NOT MOC patients presented normal CA 19.9 and CA 125 (Table 2). Both tumour markers showed similar sensitivity (50%) in MOC diagnosis and slightly higher specificity with CA 19.9 (97.6%) than with CA 125 (95.1%) (Table 3).
In some studies [12, 13], significantly higher serum CA 125 levels were found in premenopausal women than in postmenopausal women; in our case this is not significant (P>0.05). In other study, up to 61% of women with borderline ovarian tumours had elevated CA 125 [14]. In mucinous borderline ovarian tumours with papilla formation, others authors found a significant relation between elevated CA 125 [15]. In our patients, CA 125 and CA 19.9 were not statistically significantly different (P>0.05) for the diagnosis of mucinous borderline ovarian tumours.
In conclusion, preoperative CA 19.9 and CA 125 levels showed high diagnosis efficacy to predict whether a mucinous ovarian tumour is benign or malignant.
References
1. emal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010; 60: 277–300.
2. Sung PL, Chang YH, Chao KC, Chuang CM. Task Force on Systematic Review and Meta-analysis of Ovarian Cancer. Global distribution pattern of histological subtypes of epithelial ovarian cancer: a database analysis and systematic review. Gynecol Oncol. 2014; 133: 147–54.
3. Lee KR, Tavassoli FA, Prat J, et al. WHO histological classification of tumours of the ovary. In: Pathology and genetics of tumours of the breast and female genital organs. Edited by Tavassoli FA, Devilee P. IARC Press 2003; 113–161.
4. Nolen B, Marrangoni A, Velikokhatnaya L, et al. A serum based analysis of ovarian epithelial tumourigenesis. Gynecol Oncol. 2009; 112: 47–54.
5. Frumovitz M, Schmeler KM, Malpica A, et al. Unmasking the complexities of mucinous ovarian carcinoma. Gynecol Oncol. 2010; 117: 491–496.
6. Husseinzadeh N. Status of tumour markers in epithelial ovarian cancer has there been any progress? A review. Gynecol Oncol. 2011; 120: 152–157.
7. Tholander B, Taube A, Lindgren A, et al. Pretreatment serum levels of CA-125, carcinoembryonic antigen, tissue polypeptide antigen, and placental alkaline phosphatase in patients with ovarian carcinoma: influence of histological type, grade of differentiation, and clinical stage of disease. Gynecol Oncol. 1990; 39: 26–33.
8. Terzic M, Dotlic J, Likic I, et al. Diagnostic value of serum tumour markers evaluation for adnexal masses. Cent Eur J Med. 2014; 9: 210–216.
9. Gemer O, Oustinov N, Gdalevich M, et al. Pretreatment CA 15-3 levels do not predict disease-free survival in patients with advanced epithelial ovarian cancer. Tumori. 2013; 99: 257–260.
10. Kelly PJ, Archbold P, Price JH, et al. Serum CA 19.9 levels are commonly elevated in primary ovarian mucinous tumours but cannot be used to predict the histological subtype. J Clin Pathol. 2010; 63: 169–173.
11. Sturgeon CM, Duffy MJ, Walker G. The National Institute for Health and Clinical Excellence (NICE) guidelines for early detection of ovarian cancer: the pivotal role of the clinical laboratory. Ann Clin Biochem. 2011; 48: 295–299.
12. Molina R, Escudero JM, Augé JM, et al. HE4 a novel tumour marker for ovarian cancer: comparison with CA 125 and ROMA algorithm in patients with gynaecological diseases. Tumour Biol. 2011; 32: 1087–1095.
13. Escudero JM, Auge JM, Filella X, et al. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumour marker in patients with malignant and nonmalignant diseases. Clin Chem. 2011; 57: 1534–1544.
14. Morotti M, Menada MV, Gillott DJ, et al. The preoperative diagnosis of borderline ovarian tumours: a review of current literature. Arch Gynecol Obstet. 2012; 285: 1103–1112.
15. Alanbay I, Aktürk E, Coksuer H, et al. Comparison of tumour markers and clinicopathological features in serous and mucinous borderline ovarian tumours. Eur J Gynaecol Oncol. 2012; 33: 25–30.
The authors
J. D. Santotoribio1,2,*, A. Garcia-de la Torre1,2, C. Cañavate-Solano1,2, F. Arce-Matute1, M. J. Sanchez-del Pino2, S. Perez-Ramos1,2
1Clinical Biochemistry Laboratory, Puerto Real University Hospital, Cadiz, Spain
2Dept. of Biomedicine, Biotechnology and Public Health, University of Cadiz, Cadiz, Spain
*Corresponding author
E-mail: jdsantotoribioc@gmail.com