Ultrasensitive colorimetric detection of HIV-1 p24
To reduce the window period for HIV-1 infection, a method for detecting trace amounts of HIV-1 p24 in blood is needed. We developed a simple de novo ultrasensitive colorimetric ELISA by adding a thio-NAD cycling solution to the standard ELISA. The limit of detection for p24 was 0.005 IU (i.e. attomoles) per assay by the ultrasensitive colorimetric ELISA.
by Dr A. Nakatsuma, M. Kaneda, H. Kodama, M. Morikawa, S. Watabe, et al.
Background
During the window period between infection with human immunodeficiency virus type 1 (HIV-1) and the appearance of detectable antibodies to HIV-1, the infection cannot be diagnosed. Attempts to shorten this period have been made using a fourth-generation immunoassay that detects both HIV-1/2 IgG/M and HIV-1 p24 antigens [1]. However, most of the commercially available detection systems for fourth-generation immunoassays use chemiluminescent measurement and thus require specialized, highly expensive automated measurement equipment. For this reason, fourth-generation immunoassays are performed only at diagnostics companies and hub hospitals. To overcome this limitation and to test many samples simultaneously, there is need of an immunoassay with increased sensitivity for the HIV-1 p24 antigen that nonetheless uses a common enzyme and does not require any specialized instruments.
In 2010, French health authorities mandated a limit of detection of at least 2 IU/mL of HIV-1 p24 antigen for a Conformité Européenne (CE)-marked HIV antigen/antibody assay [2]. According to this mandate, commercially available assay kits were manufactured to detect p24 antigen with limits of detection ranging from 0.505 to 1.901 IU/mL and from 11.9 to 33.5 pg/mL [2]. Units of pg/mL are used for the Société Française de Tranfusion Sanguine (SFTS) standard (i.e. recombinant proteins), versus IU/mL for the WHO (World Health Organization) standard. As 1 IU/mL is estimated to be equivalent to 10 pg/mL and MW = 24 000 for p24, the best sensitivity in these kits is 0.505 IU/mL, which is ~2 × 10−16 moles/mL.
To date, numerous methods have been proposed for the detection of p24 antigen. However, the limit of detection of p24 antigen is not expected to overcome the sensitivity of 10−17 to 10−18 moles/mL. In addition, we have to note that HIV testing of many samples requires not only ultrasensitive HIV-1 p24 detection but also rapidity, a reasonable cost, and a simple protocol without the requirement of special equipment. In the present review, we introduce a de novo ultrasensitive colorimetric enzyme-linked immunosorbent assay (ELISA) for HIV-1 p24 [3].
Mechanism of ultrasensitive colorimetric ELISA
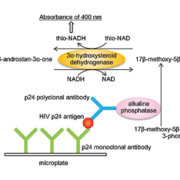
Watabe and colleagues developed an ultrasensitive ELISA to measure trace amounts of proteins by combining a conventional ELISA with thionicotinamide-adenine dinucleotide (thio-NAD) cycling [4]. Their rationale was that although proteins cannot be amplified by polymerase chain reaction (PCR) in the manner of nucleic acids, a detectable signal for proteins can be amplified. Thus, their ultrasensitive ELISA (Fig. 1) employs a sandwich method using a primary and a secondary antibody for antigens. An androsterone derivative, 3α-hydroxysteroid, is produced by the hydrolysis of 3α-hydroxysteroid 3-phosphate with alkaline phosphatase linked to the secondary antibody. This 3α-hydroxysteroid is oxidized to a 3-ketosteroid by 3α-hydroxysteroid dehydrogenase (3αHSD) with a cofactor thio-NAD. By the opposite reaction, the 3-ketosteroid is reduced to a 3α-hydroxysteroid by 3α-HSD with a cofactor NADH. During this cycling reaction, thio-NADH accumulates in a quadratic function-like fashion. Accumulated thio-NADH can be measured directly at an absorbance of 400 nm without any interference from other cofactors.
This method enables the detection of a target protein with ultrasensitivity (10−19 moles/assay) by measuring the cumulative quantity of thio-NADH by a colorimetric method without the use of any special instruments for the measurements of fluorescence, luminescence or radio isotopes [4]. Further, we should note that this ultrasensitive method will allow a technician to detect trace amounts of proteins simply by applying thio-NAD cycling reagents to the conventional ELISA system. We therefore applied this ultrasensitive ELISA to the detection of HIV-1 p24 antigen in blood [3].
Sensitivity and stability of the ultrasensitive colorimetric ELISA for HIV-1 p24
A typical linear calibration curve for HIV-1 p24 antigen provided by the ultrasensitive ELISA coupled with thio-NAD cycling was y = 0.27x + 0.019, R2 = 0.99 in the range of 0.1‒1.0 IU/mL. The limit of detection of p24 was 0.0055 IU/assay (i.e. ~2 × 10−18 moles/assay). These findings indicate that the ultrasensitive colorimetric ELISA succeeds in detecting p24 at the attomole level [3]. Because this measurement system employs a 50 µL solution for each assay, the detection limit corresponded to 0.1 IU/mL, or 10−17 moles/mL. Therefore, even in terms of the concentration per mL, our detection limit is less than one-tenth of that required by the French health authorities [2]. The coefficient of variation was 8% for 1 IU/mL.
Spike-and-recovery test using serum
We attempted to perform spike-and-recovery tests in which the HIV-1 p24 antigen was added to the control serum. Because our results demonstrated that the ratio was about 100% for 0.5 IU/mL of HIV-1 p24, which was less than the value (2 IU/mL) required for a CE-marked HIV antigen/antibody assay (see Background), the ultrasensitive method was judged to sufficiently detect IV-1 p24 antigen in human blood obtained from patients in the very early period after infection.
Detection of HIV-1 p24 in the early stages of infection
It is important to diagnose primary HIV-1 infection and begin antiretroviral treatment as early as possible. Most HIV-1/2 antibody diagnostic tests detect the antibodies for the antigens of HIV-1 gp41 and HIV-2 gp36, which are highly conservative transmembrane proteins. These tests are quick and easy, and thus have been widely used in many clinics and public health centres. However, when only the antibody diagnostic tests are used, there is a long delay (generally a 28-day window period) before diagnosis is possible [5]. Further, HIV-1/2 antibody tests in children younger than 18 months tend to be especially inaccurate as a result of the continued presence of maternal antibodies [6]. To shorten the delay and to validate HIV tests, the HIV-1 p24 antigen, the concentration of which is expected to increase before antibodies emerge, should be detectable in trace amounts. HIV-1 p24 in blood emerges transiently in the very early period after infection, and then its concentration quickly returns to the basal level [5]. An HIV-1 p24 test is, therefore, very useful as a screening test in the early stage of infection.
Closing the gap on PCR-based nucleic acid testing (NAT)
Generally, the gold standard for diagnosing HIV-1 is PCR-based nucleic acid testing (NAT) [7], but this method is expensive and has infrastructure requirements, a long measuring time, and high complexity, thereby limiting its usefulness for large numbers of samples. There is also the issue that much of the world lacks access to reliable NAT, and thus in many geographic regions the policy is to simply wait until symptoms develop. Use of ultrasensitive detection of HIV-1 p24 antigen for early diagnosis would be a simple and reasonable alternative to NAT, such as for monitoring HIV treatment and protecting the blood supply. Accordingly, it is time to reconsider whether NAT should be the gold standard for diagnosing HIV-1. Barletta et al. claimed that the target protein (i.e. HIV-1 p24 antigen) is present in the virion in much higher numbers than viral RNA copies (approximately 3000 HIV-1 p24 antigen molecules versus 2 RNA copies per virion) [8]. The 10−18 moles/assay value in our present results corresponds to 106 protein molecules/assay, or ~103 RNA copies/assay. Although under laboratory conditions a real-time PCR (i.e. NAT) can detect on the order of 101 RNA copies/assay, the limitation of detection is usually in the order of 102 RNA copies/assay [9]. Hence, the ultrasensitive ELISA coupled with thio-NAD cycling for HIV-1 p24 is closing in on the detection limit obtained by NAT, with a margin of difference of only one order of magnitude.
Conclusion
The ultrasensitive ELISA coupled with thio-NAD cycling is a very convenient method for the early testing of HIV-1 infection because it requires only the addition of a thio-NAD cycling solution to the usual ELISA without the use of any specialized measuring equipment. Consequently, the present method could be widely used as a powerful tool to test many samples simultaneously.
References
1. George CRR, Robertson PW, Lusk MJ, Whybin R, Rawlinson W. Prolonged second diagnostic window for human immunodeficiency virus type 1 in a fourth-generation immunoassay: Are alternative testing strategies required? J Clin Microbiol. 2014; 52: 4105–4108.
2. Ly TD, Plantier JC, Leballais L, Gonzalo S, Lemée V, Laperche S. The variable sensitivity of HIV Ag/Ab combination assays in the detection of p24Ag according to genotype could compromise the diagnosis of early HIV infection. J Clin Virol. 2012; 55: 121–127.
3. Nakatsuma A, Kaneda M, Kodama H, Morikawa M, Watabe S, Nakaishi K, Yamashita M, Yoshimura T, Miura T, Ninomiya M, Ito E. Detection of HIV-1 p24 at attomole level by ultrasensitive ELISA with thio-NAD cycling. PLoS One 2015; 10: e0131319.
4. Watabe S, Kodama H, Kaneda M, Morikawa M, Nakaishi K, Yoshimura T. Ultrasensitive enzyme-linked immunosorbent assay (ELISA) of proteins by combination with the thio-NAD cycling method. BIOPHYSICS. 2014; 10: 49–54.
5. World Health Organization (WHO). HIV/AIDS Fact sheet No 360. WHO 2015; http://www.who.int/mediacentre/factsheets/fs360/en/
6. Zijenah LS, Tobaiwa O, Rusakaniko S, Nathoo KJ, Nhembe M, Matibe P, Katzenstein DA. Signal-boosted qualitative ultrasensitive p24 antigen assay for diagnosis of subtype C HIV-1 infection in infants under the age of 2 years. J Acquir Immune Defic Syndr. 2005; 39: 391–394.
7. Patel P, Mackellar D, Simmons P, Uniyal A, Gallagher K, Bennett B, Sullivan TJ, Kowalski A, Parker MM, LaLota M, Kerndt P, Sullivan PS; Centers for Disease Control and Prevention Acute HIV Infection Study Group. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006-2008. Arch Intern Med. 2010; 170: 66–74.
8. Barletta JM, Edelman DC, Constantine NT. Lowering the detection limits of HIV-1 viral load using real-time immuno-PCR for HIV-1 p24 antigen. Am J Clin Pathol. 2004; 122: 20–27.
9. Wagatsuma A, Sadamoto H, Kitahashi T, Lukowiak K, Urano A, Ito E. Determination of the exact copy numbers of particular mRNAs in a single cell by quantitative real-time RT-PCR. J Exp Biol. 2005; 208: 2389–2398.
The authors
Akira Nakatsuma1 PhD, PhC; Mugiho Kaneda1 BAgr; Hiromi Kodama1 MAgr; Mika Morikawa1,2 BASc; Satoshi Watabe3 BPha; Kazunari Nakaishi2; Masakane Yamashita4 PhD; Teruki Yoshimura5 PhD, PhC; Toshiaki Miura6 PhD, PhC; Masaki Ninomiya1 PhD, PhC; Etsuro Ito*1 PhD
1 Kagawa School of Pharmaceutical Sciences, Tokushima Bunri University, Sanuki, Japan
2 TAUNS Laboratories, Inc., Izunokuni, Japan
3 BL Co., Ltd., Numazu, Japan
4 Faculty of Science, Hokkaido University, Sapporo, Japan
5 Faculty of Pharmaceutical Sciences, Health Sciences University of Hokkaido, Ishikari-Tobetsu, Japan
6 Graduate School of Pharmaceutical Sciences, Hokkaido University, Sapporo, Japan
*Corresponding author
E-mail: eito@kph.bunri-u.ac.jp