Uncovering molecular biomarkers in gastric adenocarcinoma
Gastric adenocarcinoma is usually diagnosed at an advanced stage, which portends a poor prognosis. Molecular biomarkers are important tools to understand the underlying biology of its aggressive behaviour and to discover new targets for therapeutic agents. Microarray analyses and next generation sequencing are leading to a deeper understanding of tumour biology and the development of new biomarkers, offering hope for better treatment approaches in the future.
by Dr I. Snitcovsky, Dr F. Solange Pasini and Dr G. de Castro Jr
Background
Gastric cancer is the fourth most common cancer in the world and is especially prevalent in East Asia and South America [1]. Adenocarcinoma accounts for the great majority of these tumours, which are classified as intestinal or diffuse type. The pathogenesis is incompletely understood, but it is associated with Helicobacter pylori infection and dietary salt and nitrosamines, particularly in intestinal type tumours. In these cases, chronic inflammation is thought to lead to preneoplastic lesions that may progress to invasive cancer in a stepwise fashion. In a minority of cases, germline mutations of P53, CDH1 and mismatch repair genes are associated with familial cases. The most important prognostic factor is the tumour TNM stage, since the only curative approach is surgical resection, followed (or not) by adjuvant therapies. Thus, locally advanced and metastatic disease portends a poor prognosis, with a median survival of less than one year. Unfortunately, most patients in Western countries are diagnosed with advanced disease, and, in these cases, chemotherapy can palliate symptoms and prolong overall survival but it is not curative [2]. In patients with metastatic disease, the only biomarker routinely tested for in gastric adenocarcinoma is HER2 (human epidermal growth factor 2 receptor; by immunohistochemistry), which is associated with poor outcomes and is also predictive of the anti-tumour efficacy of the humanized anti-HER2 antibody, trastuzumab [3].
The development of innovative treatment approaches begins with the identification of molecular biomarkers relevant to tumour biology. The next step is clinical validation, usually by showing that the studied biomarker has a prognostic value. Finally, a targeted agent is developed and shown to prolong survival in phase III clinical trials. Genome-wide studies are revealing potential biomarkers for targeted therapies and immunotherapy. This review will focus on recently identified candidate biomarkers in gastric adenocarcinoma with potential clinical applications.
Biomarkers in the pre-genomic era
Cancer cells are characterized by self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of apoptosis, limitless reproductive potential, sustained angiogenesis and tissue invasion and metastasis [4]. Accordingly, in gastric adenocarcinoma, a great number of studies focused on the prognostic role of single molecules. They included, but were not restricted to, growth factors and their receptors [HER2, IGFR (insulin-like growth factor 1 receptor)], cell cycle regulators (p53), angiogenesis controllers [VEGF (vascular endothelial growth factor)] and matrix metalloproteinases, with so far no impact in patient management, with the exception of HER2.
The epidermal growth factor receptor (EGFR) family includes HER1 (EGFR), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4). These molecules form dimers on the cell surface after ligand binding, which leads to intracellular signalling that modulates cell proliferation, metastasis and angiogenesis. HER2 has no known ligands, but forms heterodimers with other members of the HER family and potentiates signalling. In breast cancer, HER2 overexpression is related to poor prognosis and the humanized anti-HER2 monoclonal antibody trastuzumab prolongs survival in those patients with HER2 positive tumours [5]. In gastric adenocarcinoma, HER2 overexpression is detected in 9–35% of cases and implies a worse prognosis in some studies. A phase III trial that included patients with HER2-overexpressing gastric adenocarcinoma found that the addition of trastuzumab to chemotherapy resulted in an overall survival benefit of about two months, as compared to chemotherapy alone [3]. In contrast, phase III studies evaluating anti-angiogenic agents in unselected gastric adenocarcinoma patients presented conflicting results [6, 7].
Gene panels and next generation sequencing
Gastric adenocarcinoma is a heterogeneous disease, thus the simultaneous determination of several biomarkers may be more informative then single ones, nowadays possible by high-throughput technologies as microarray platforms and next generation sequencing. Chen et al. [8] proposed a prognostic three-gene model, derived from gene expression profiling in eighteen paired samples. Marchet et al. [9] proposed another three-gene model predictive of lymph node involvement in a cohort of 32 patients. Another prognostic four-gene signature was also described [10]. Little overlap was observed among these above-mentioned signatures, which is not informative in terms of advancing in the cancer biomarker development.
A study comparing 248 gastric adenocarcinoma tumour samples was able to classify tumours in three subtypes, based on gene expression patterns: proliferative, metabolic and mesenchymal. In addition, these subtypes were shown to have differences in molecular and genetic features, and response to therapy [11]. Next generation sequencing is providing a deeper level of understanding the tumour biology. Genetic alterations were observed in Wnt, Hedgehog, cell cycle, DNA damage and epithelial-to-mesenchymal-transition pathways by analysing the genome and the transcriptome in 50 adenocarcinoma samples. About 20% of these alterations could be considered as potential targets for drugs that are already available [12]. Novel fusion genes were identified, especially DUS4L-BCAP29, when transcriptome sequencing was performed in 12 gastric adenocarcinoma cell lines. Knockdown of this transcript inhibited cell proliferation, thus validating its functional role [13].
Immune biomarkers
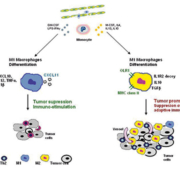
Cancer, including gastric adenocarcinoma, is viewed as a tissue disease. This implies that the microenvironment plays a key role in tumour biology [4]. Thus, immune cell infiltrate has been shown to be of prognostic value in gastric adenocarcinoma. As depicted in Figure 1, tumour-associated macrophages present two different polarizations: classical (M1) characterized by immunostimulation activity and tumour suppression; and alternative (M2) characterized by tumour promotion and immune suppression. A higher ratio of M1/M2 macrophages was associated with a favourable prognosis [14]. The underlying mechanism is complex, but may involve growth factor modulation [15]. We conducted a gene expression study, including a total of 51 freshly frozen tumour samples from patients with gastric adenocarcinoma treated with surgery. An immune-related gene trio (OLR1, CXCL11 and ADAMDEC1) was identified as an independent biomarker of prognosis. We proposed that immune dampening in the tumour microenvironment was present in patients with poor prognosis. Three main observations supported our hypothesis. First, the expression levels of genes belonging to the functional group of immune/inflammatory response were markedly reduced as a whole. Second, a network analysis suggested an unwired inflammatory response, and third, a decreased expression of type-1 helper lymphocyte (Th1) and other immune activating genes was found [16]. The biomarkers we identified may be good candidates for selecting patients for immunomodulation therapies, including immune checkpoint inhibitors [17].
Conclusions and perspectives
Gastric adenocarcinoma needs better treatment approaches. New technologies are offering the necessary tools to identify molecular biomarkers, leading to a deeper understanding of tumour biology and the development of innovative treatment strategies, and we are entering an era of cautious optimism. Considering the tumour heterogeneity and the limited survival gains with targeted agents in solid tumours, it is possible that patient selection by immune biomarkers and the use of immune checkpoint inhibitors are promising alternatives. The impressive response rates and overall survival benefits observed in patients with squamous cell lung cancer and melanoma, two notoriously chemoresistant tumours, when treated with anti-PD1 or anti-PD1L are good examples [18].
References
1. Ferlay J, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11; 2013. (http://globocan.iarc.fr)
2. Waddell T, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):57.
3. Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer. Lancet 2010;376: 687.
4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646.
5. Figueroa-Magalhães MC, et al. Treatment of HER2- positive breast cancer. Breast 2013;doi:10.1016/j.breast.2013.11.011.
6. Ohtsu A, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968.
7. Fuchs CS, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31.
8. Chen CN, et al. Gene expression profile predicts patient survival of gastric cancer after surgical resection. J Clin Oncol. 2005;23:7286.
9. Marchet A, et al. Gene expression profile of primary gastric cancer: towards the prediction of lymph node status. Ann Surg Oncol. 2007;14:1058.
10. Xu ZY, et al. Gene expression profile towards the prediction of patient survival of gastric cancer. Biomed Pharmacother. 2010;64:133.
11. Lei Z, et al. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology 2013; 145:554.
12. Holbrook JD, et al. Deep sequencing of gastric carcinoma reveals somatic mutations relevant to personalized medicine. J Transl Med. 2011;9:119.
13. Kim HP, et al. Novel fusion transcripts in human gastric cancer revealed by transcriptome analysis. Oncogene 2013;doi:10.1038/onc.2013.490.
14. Pantano F, et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J Cell Mol Med. 2013;17:1415.
15. Cardoso AP, et al. Macrophages stimulate gastric and colorectal cancer invasion through EGFR Y(1086), c-Src, Erk1/2 and Akt phosphorylation and smallGTPase activity. Oncogene 2013;doi:10.1038/onc.2013.154.
16. Pasini FS, et al. A gene expression profile related to immune dampening in the tumor microenvironment is associated with poor prognosis in gastric adenocarcinoma. J Gastroenterol. 2013;doi:10.1007/s00535-013-0904-0.
17. Eggermont AM, et al. Immunotherapy and the concept of a clinical cure. Eur J Cancer 2013;49: 2965.
18. Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455.
The authors
Igor Snitcovsky1,2 MD, PhD; Fátima Solange Pasini1,2 PhD; and Gilberto de Castro Jr*1,3 MD, PhD
1 Departamento de Radiologia e Oncologia, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil
2 Centro de Investigação Translacional em Oncologia, Instituto de Câncer do Estado de São Paulo (ICESP), São Paulo, Brazil
3 Oncologia Clínica, Instituto do Câncer do Estado de São Paulo (ICESP), São Paulo, Brazil
*Corresponding author
E-mail: gilberto.castro@usp.br