Use of global hemostatic markers for risk stratification and personalized treatment of coronary artery disease
Coronary artery disease has been linked to a hypercoagulable state of the blood, and the use of global hemostatic assays such as thromboelastography, thrombin generation or the overall hemostatic assay may allow for prediction of adverse events in these patients as well as targeted, individualized treatment.
by Dr C. Reddel, Dr J. Curnow and Professor D. Brieger
Global hemostatic markers in coronary artery disease
Hemostasis is the process by which bleeding is stopped, involving blood coagulation and platelet aggregation. This process depends on the delicate balance of many pro- and anti-coagulant factors, and when hemostatic balance is disrupted, pathological clot formation may occur leading to potentially fatal venous or arterial thrombosis. Appropriate fibrinolysis, the breakdown of blood clots, is also essential to the process of hemostasis.
Coronary artery disease is considered an inflammatory disease in which patients are predisposed to arterial thrombosis, which can lead to myocardial infarction. Additionally, the presence of coronary artery disease can increase the risk of venous thrombosis [1]. This points to an overall hypercoagulable state of the blood in this disease. Although the use of antiplatelet and anticoagulant therapies is a common and necessary method of reducing this risk, this may unnecessarily expose patients to a risk of bleeding. There is a need to risk stratify patients and individually tailor thromboprophylaxis.
Imbalances in the hemostatic system can be assessed in citrated plasma samples from patients either by measuring individual coagulation and fibrinolytic factors, or by global coagulation assays. Such imbalances have been found to be associated with various pro-thrombotic states, such as cancer, pregnancy or trauma. In stable and acute coronary artery disease, there is evidence for links between prognosis and markers of coagulation and fibrinolysis, including prothrombin fragment 1+2, fibrinopeptide A, thrombin–antithrombin and plasmin–antiplasmin complexes, D-dimer, plasminogen activator inhibitor-1, thrombin activatable fibrinolysis inhibitor and tissue plasminogen activator [2, 3]. However, measuring single factors does not reflect the overall hemostatic balance as other pro- or anti-coagulant, and pro- and anti-fibrinolytic factors may compensate for the deficient or elevated factor. Therefore measurement of the overall coagulable state of the blood may provide a more relevant picture.
Standard laboratory coagulation tests, such as prothrombin time (PT) or activated partial thromboplastin time (APTT), can be useful for patients with bleeding disorders, but do not reliably detect hypercoagulability in this context. Recently, there has been interest in global assays of coagulation and fibrinolysis as methods of assessing the overall potential of a patient’s blood to form or lyse a clot. These include assays of thrombin generation, thromboelastography and the overall hemostatic potential assay.
Thromboelastography
Thromboelastography is a method measuring clot formation and lysis in whole blood. A pin is suspended into a cuvette of whole blood heated to 37°C, and the cup and pin move relative to each other, so that when the clot forms the interference is detected by the pin. Thromboelastography (TEG, Haemonetics, Braintree, Massachusetts, USA) and Thromboelastometry (ROTEM, Tem International GmbH, Munich, Germany) are two commercial variants of the assay. The assay measures not only time to clot, but speed of clot formation, clot strength and elasticity, and can be modified to assess platelet function, fibrinogen, hyperfibrinolysis and effect of anticoagulant treatment. The use of whole blood means the role of the cell is incorporated into the assay, although this necessitates immediate use of the sample.
Thromboelastography is a point-of-care assay which is used to measure and characterize peri-operative bleeding. It may additionally be useful in monitoring antiplatelet therapy such as aspirin or clopidogrel. Recently, it has also been used to detect hypercoagulability in patients with coronary artery disease, and further, has been demonstrated to predict thrombotic events in patients who have undergone coronary stenting or coronary artery bypass grafting [4, 5].
Thrombin generation assay
The thrombin generation assay was first described in 1953, but has more recently been simplified, standardized and commercialized, including in the form of the Calibrated Automated Thrombogram (Thrombinoscope BV, Maastricht, The Netherlands) and Technothrombin (TGA, Technoclone, Vienna, Austria) [6]. In this assay, ex vivo potential for thrombin generation is measured in platelet-rich or platelet-poor plasma. In a 96-well plate, thrombin generation is triggered by addition of tissue factor, phospholipids and calcium at 37°C, and conversion of a substrate for thrombin measured over an hour by fluorescence.
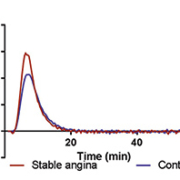
Thrombin is central to the process of hemostasis, and various pro-thrombotic states have been associated with variations in plasma potential to generate thrombin. Patients with stable coronary artery disease have elevated thrombin generation [Fig. 1] [7], and patients with acute coronary syndrome have still higher thrombin potential [8]. Antiplatelet therapies most likely do not affect the thrombin generation assay in platelet-poor plasma, but it may be possible to monitor the effect of anticoagulant drugs (including novel oral anticoagulants) using the assay, and preliminary assessment has suggested the assay can predict bleeding and ischemic events in patients with coronary artery disease [9].
Overall Hemostatic Potential (OHP) assay
The Overall Hemostatic Potential (OHP) assay is a test of fibrin generation and fibrinolysis first described in 1999 [10]. Similar to the thrombin generation assay, it is performed in citrated plasma in 96-well plates and triggered by tissue factor or thrombin and calcium at 37°C. It is a turbidometric assay, measuring the change in absorbance over an hour at 405nm, which allows for a kinetic analysis of fibrin clot formation. Tissue plasminogen activator is also added to half the wells, which triggers fibrinolysis. The assay measures coagulation potential and fibrinolytic potential, and is carried out on stored plasma samples.
A limitation of the plasma-based thrombin generation and OHP assays is the absence of cells. These assays have nonetheless identified differences between patients with pro-thrombotic states and healthy controls, and the use of plasma allows for samples to be stored and batch-tested, which is an advantage for screening large numbers of patients. The OHP assay additionally requires no specialized equipment, apart from a standard plate reader, and although not standardized, it is inexpensive. Unlike thromboelastography which is relatively insensitive to hypofibrinolysis, the OHP assay can detect and quantify hypofibrinolysis as well as hyperfibrinolysis.
Very recently the OHP assay has been used to show hypercoagulability and hypofibrinolysis in patients with acute and stable coronary artery disease [Fig. 2] [7, 11]. The observations in this latter population suggest the potential for this assay to predict future events, and prospective studies are required to determine its utility in this context.
Future trends and requirements
There is a growing body of evidence that ex vivo hypercoagulability of patients’ blood or plasma has prognostic value in arterial or venous thrombotic events. Global markers of hemostasis, including results of thromboelastography, the thrombin generation and OHP assays, may prove clinically relevant in identifying individual patients at risk of adverse event, and thus allow the tailoring of thromboprophylaxis. Further large-scale prospective trials are needed to directly address this.
References
1. Anandasundaram B, Lane DA, Apostolakis S, Lip GY. The impact of atherosclerotic vascular disease in predicting a stroke, thromboembolism and mortality in atrial fibrillation patients: a systematic review. J Thromb Haemost. 2013; 11: 975–987.
2. Stegnar M, Vene N, Bozic M. Do haemostasis activation markers that predict cardiovascular disease exist? Pathophysiol Haemost Thromb. 2003; 33: 302–308.
3. Gorog DA. Prognostic value of plasma fibrinolysis activation markers in cardiovascular disease. J Am Coll Cardiol. 2010; 55:2 701–709.
4. Hobson AR, Agarwala RA, Swallow RA, Dawkins KD, Curzen NP. Thrombelastography: current clinical applications and its potential role in interventional cardiology. Platelets 2006; 17: 509–518.
5. McCrath DJ, Cerboni E, Frumento RJ, Hirsh AL, Bennett-Guerrero E. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005; 100: 1576–1583.
6. Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002; 32: 249–253.
7. Reddel CJ, Curnow JL, Voitl J, Rosenov A, Pennings GJ, Morel-Kopp MC, et al. Detection of hypofibrinolysis in stable coronary artery disease using the overall haemostatic potential assay. Thromb Res. 2013; 131: 457–462.
8. Orbe J, Zudaire M, Serrano R, Coma-Canella I, Martinez de Sizarrondo S, Rodriguez JA, et al. Increased thrombin generation after acute versus chronic coronary disease as assessed by the thrombin generation test. Thromb Haemost. 2008; 99: 382–327.
9. Campo G, Pavasini R, Pollina A, Fileti L, Marchesini J, Tebaldi M, et al. Thrombin generation assay: a new tool to predict and optimize clinical outcome in cardiovascular patients? Blood Coag Fibrinolysis 2012; 23: 680-687.
10. He S, Bremme K, Blomback M. A laboratory method for determination of overall haemostatic potential in plasma. I. Method design and preliminary results. Thromb Res. 1999; 96: 145–156.
11. Leander K, Blomback M, Wallen H, He S. Impaired fibrinolytic capacity and increased fibrin formation associate with myocardial infarction. Thromb Haemost. 2012; 107: 1092–1099.
The authors
Caroline Reddel* PhD; Jennifer Curnow MBBS, PhD, FRACP, FRCPA; David Brieger MBBS, PhD, FRACP, FACC
ANZAC Research Institute, Concord Repatriation General Hospital, Concord NSW, 2139, Australia
*Corresponding author
E-mail: creddel@anzac.edu.au