Variability of the response to clopidogrel: mechanisms, availability of testing, and relation to clinical outcomes
Interindividual variability in the response to clopidogrel has been shown to be related to the clinical ischemic outcomes. Although testing of platelet function or genetic profile is recommended to evaluate the response to clopidogrel, standardized testing and definitive antiplatelet therapy after testing need to be established.
by Yusuke Yamaguchi and Professor Mitsuru Murata
Clinical background
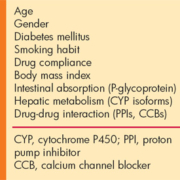
Platelet activation and aggregation play a pivotal role in arterial thrombosis formation; therefore, antiplatelet therapy to inhibit platelet function is considered effective for preventing and treating atherothrombosis. The combination of aspirin and clopidogrel has been shown to be more effective than aspirin alone for improving clinical ischemic outcomes in patients with coronary artery disease (CAD). This dual antiplatelet therapy contributes substantially to prevent the occurrence of cardiovascular events in patients with acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI). Current guidelines recommend aspirin and clopidogrel for these patients; however, some patients still develop cardiovascular events despite dual therapy. It has been shown in the last decade that the responsiveness to clopidogrel is highly variable in individuals and that a suboptimal response to clopidogrel is a risk factor for cardiovascular events. The interindividual variability in the effect of clopidogrel is due to multiple factors [Table 1].
Effects of CYP2C19 on clopidogrel
Clopidogrel, a second generation thienopyridine, is an inactive prodrug that requires a 2-step metabolic conversion to an active metabolite. This active metabolite inhibits adenosine diphosphate (ADP)-induced platelet aggregation by selectively and irreversibly binding P2Y12 receptors on the platelet membrane. Several isoforms of cytochrome P450 (CYP), including CYP2C19, CYP3A4, CYP1A2, CYP2B6, and CYP2C9, have been shown to be involved in the metabolic pathway. Of these enzymes, CYP2C19 is considered to be the main determinant of clopidogrel metabolism that produces the active form.
It is known that CYP2C19 has numerous single nucleotide polymorphisms (SNPs), of which CYP2C19*2 (681G>A, located in exon 5) has been studied extensively and shown to be associated with a loss of function of the enzyme. CYP2C19*2 clearly associates with both the pharmacokinetics (i.e., area under the concentration curve and maximal plasma concentration of clopidogrel active metabolite) and the pharmacodynamics (i.e., inhibition of ADP-induced platelet aggregation) of clopidogrel. CYP2C19*2 is detected more frequently in Asians than in Caucasians, with approximately 40–50% and 30% having at least one CYP2C19*2 allele, respectively. In addition to CYP2C19*2, CYP2C19*3, *4, *5, *6, *7, and *8 have been identified as loss-of-function alleles.
Methods to evaluate the effect of clopidogrel on platelet inhibition
Different laboratory tests [Table 2] can be used to assess platelet function in patients treated with clopidogrel. ADP-induced platelet aggregation in platelet-rich plasma measured by light transmission aggregometry is used most commonly, with numerous published studies using this method to measure platelet function. The majority of these studies measured platelet function as maximal platelet aggregation rate induced by 5, 10, or 20 µmol/l ADP. The platelet aggregation rate 5–8 min after the addition of ADP has also been reported. The POPULAR study [1] on clopidogrel-treated patients following elective PCI showed that 42.9% maximal platelet aggregation rate induced by 5 µmol/l ADP or 64.5% induced by 20 µmol/l ADP correlated with the 1-year mortality rate, myocardial infarction (MI), stent thrombosis, and stroke.
The VerifyNow P2Y12 test (Accumetrics Inc, SanDiego, CA) has been developed as a point-of-care device to quickly and accurately assess platelet function in patients. This test is a whole-blood, light transmission-based optical detection assay that measures the light transmittance of ADP-induced platelet aggregation in a cartridge containing fibrinogen-coated beads and is able to specifically evaluate P2Y12 receptor inhibition. The results are reported as P2Y12 reaction units (PRU), with a lower PRU value being associated with higher P2Y12 inhibition. A meta-analysis of individual patient data in six observational studies [2] revealed that a PRU value of 230 at PCI is the best cut-off value for predicting the occurrence of cardiovascular events, including death, MI, and stent thrombosis, in patients with stable CAD or non-ST elevated ACS undergoing PCI over 1 year.
The effect of clopidogrel on platelet function can be also evaluated by detecting vasodilator-stimulated phosphoprotein (VASP). VASP is phosphorylated by cyclic adenosine monophosphate (cAMP) produced in the adenylate cyclase cascade downstream of the P2Y12 receptor. By binding to the P2Y12 receptor and suppressing the cascade, ADP leads to an increase in VASP dephosphorylation, whereas inhibition of the receptor by clopidogrel active metabolite leads to an increase in VASP phosphorylation. This test measures VASP phosphorylation in a flow cytometric assay with the result expressed as platelet reactivity index (PRI) that represents the ratio of the phosphorylated and dephosphorylated VASP. A lower PRI value reflects higher P2Y12 inhibition.
Clinical utility of laboratory testing
Numerous studies, including our meta-analysis [3], have reported that patients with a suboptimal response to antiplatelet therapy have increased cardiovascular events [Figure 1A], and data have been accumulated on testing of platelet function to establish a reliable cut-off value for clinical risk. However, it remains unclear how to monitor suboptimal responses in daily clinical practice due to the lack of a standardised method to measure and interpret the results of platelet function. Furthermore, there is no guideline for alternative treatment strategies to the “one-size-fits-all” 75 mg/day clopidogrel regime because conclusive evidence that personalised antiplatelet therapy improves patient outcomes has not been established from large-scale randomised trials. However, a meta-analysis [4] recently reported the evaluation of the clinical efficacy and safety of intensified antiplatelet therapy involving reloading clopidogrel, using glycoprotein IIb/IIIa inhibitors periprocedural PCI, increasing the maintenance dose of clopidogrel, or switching to prasugrel. Although there were several limitations, this meta-analysis showed that intensified antiplatelet therapy reduces cardiovascular death and stent thrombosis without increasing major bleeding.
Meanwhile, CYP2C19 genotype does not always seem to predict cardiovascular events, although it is a major predictor for suboptimal response to clopidogrel. To date, many large-scale clinical trials, including the recent Genotype Information and Functional Testing (GIFT) trial [5], which investigate an association of CYP2C19 genotype with cardiovascular events, have been performed. However, the results of these trials were inconsistent. Indeed, we showed heterogeneity in the odds ratio of the cardiovascular events between the carriers and non-carriers of CYP2C19*2 allele in our meta-analysis [Figure 1B]. Considering that CYP2C19*2 contributes to only about 5% of the variability in response to clopidogrel [6], many other genetic factors may contribute to the variability apart from CYP2C19. Therefore, genetic testing including additional factors such as SNPs in other CYPs or ABCB1 (encoding p-glycoprotein) would be expected to improve identification of patients with a suboptimal response.
Current status and future prospects
In 2009, the U.S. Food and Drug Administration (FDA) released a black box warning that significant attention needs to be paid to clopidogrel pharmacogenomics. Similarly, the American and European guidelines published in 2011 gave a Class IIb recommendation for testing of platelet function or genetic profile in patients treated with clopidogrel and for consideration of the use of an alternate P2Y12 inhibitor in patients with inadequate platelet inhibition.
The primary goal of testing of platelet function and genetic profile is to identify patients with a suboptimal response to antiplatelet therapy and provide them with a tailor therapy to improve the clinical ischemic outcomes without an
associated bleeding risk. Although these laboratory tests provide sufficient evidence to predict outcomes, personalised antiplatelet therapy on the basis of these tests has not been established in the guidelines. Currently, several clinical trials are ongoing that evaluate the effect of personalised antiplatelet therapy on the basis of laboratory tests. These trials will hopefully provide important data to establish guidelines, to allow clinicians to properly select laboratory tests, and to plan personalised antiplatelet therapy in patients with a suboptimal response.
References
1. Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, et al. JAMA 2010; 303: 754–762.
2. Brar SS, ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS, et al. J Am Coll Cardiol 2011; 58: 1945–1954.
3. Yamaguchi Y, Abe T, Sato Y, Matsubara Y, Moriki T, Murata M. Platelets. Epub 2012 Jul 3, doi: 10.3109/09537104.2012.700969
4. Aradi D, Komócsi A, Price MJ, Cuisset T, Ari H, Hazarbasanov D, et al. Int J Cardiol. Epub 2012 Jun 15, doi: 10.1016/j.ijcard.2012.05.100
5. Price MJ, Murray SS, Angiolillo DJ, Lillie E, Smith EN, Tisch RL, et al. J Am Coll Cardiol 2012; 59: 1928–1937.
6. Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, et al. J Am Coll Cardiol 2010; 55: 2427–2434.
The authors
Yusuke Yamaguchi and Mitsuru Murata MD, PhD
Dept of Laboratory Medicine, Keio University School of Medicine,
35 Shinanomachi, Shinjuku-ku, Tokyo, 160-8582, Japan
E-mail: yusukeyamaguchi@z8.keio.jp