With a little help from the patients: Automated measurement of tacrolimus from dried blood spots
Therapeutic drug monitoring (TDM) of tacrolimus is mandatory because this immunosuppressive drug that is frequently prescribed in transplant patients, exhibits a high interpatient pharmacokinetic variability. This study explored the possibility of performing TDM using dried blood spots that can be collected by the patients themselves and that can be analysed by a fully-automated extraction module coupled to a liquid chromatography-tandem mass spectrometry system.
by Gauthier Rosé
Background
Tacrolimus (TAC) is one of the most prescribed immunosuppressive drugs for transplant patients. It is characterized by a narrow therapeutic index and high intra and inter-individual pharmacokinetics variability [1]. For these reasons, therapeutic drug monitoring (TDM) is mandatory for TAC for transplant patients. Currently, TDM is performed using whole blood samples (WB), and liquid chromatography-tandem mass spectrometry (LC-MS/MS) has been established as the gold standard for this purpose [2].
However, a growing interest in home-based micro-sampling has led to the use of dried blood spot (DBS) specimens as an alternative to WB [3]. Preparation of a high number of DBS samples requires time-consuming extraction by technicians, incurring the need for automation.
Shimadzu’s CLAM-2030 is a module that automates all analytical process steps and is connected directly to a liquid-chromatography system to enable seamless analysis and management of samples (Fig. 1). Today, the module can autonomously handle various types of wet samples (WB, urine, serum or plasma) [4, 5]; such automation has not yet been employed to prepare solid samples such as DBS.
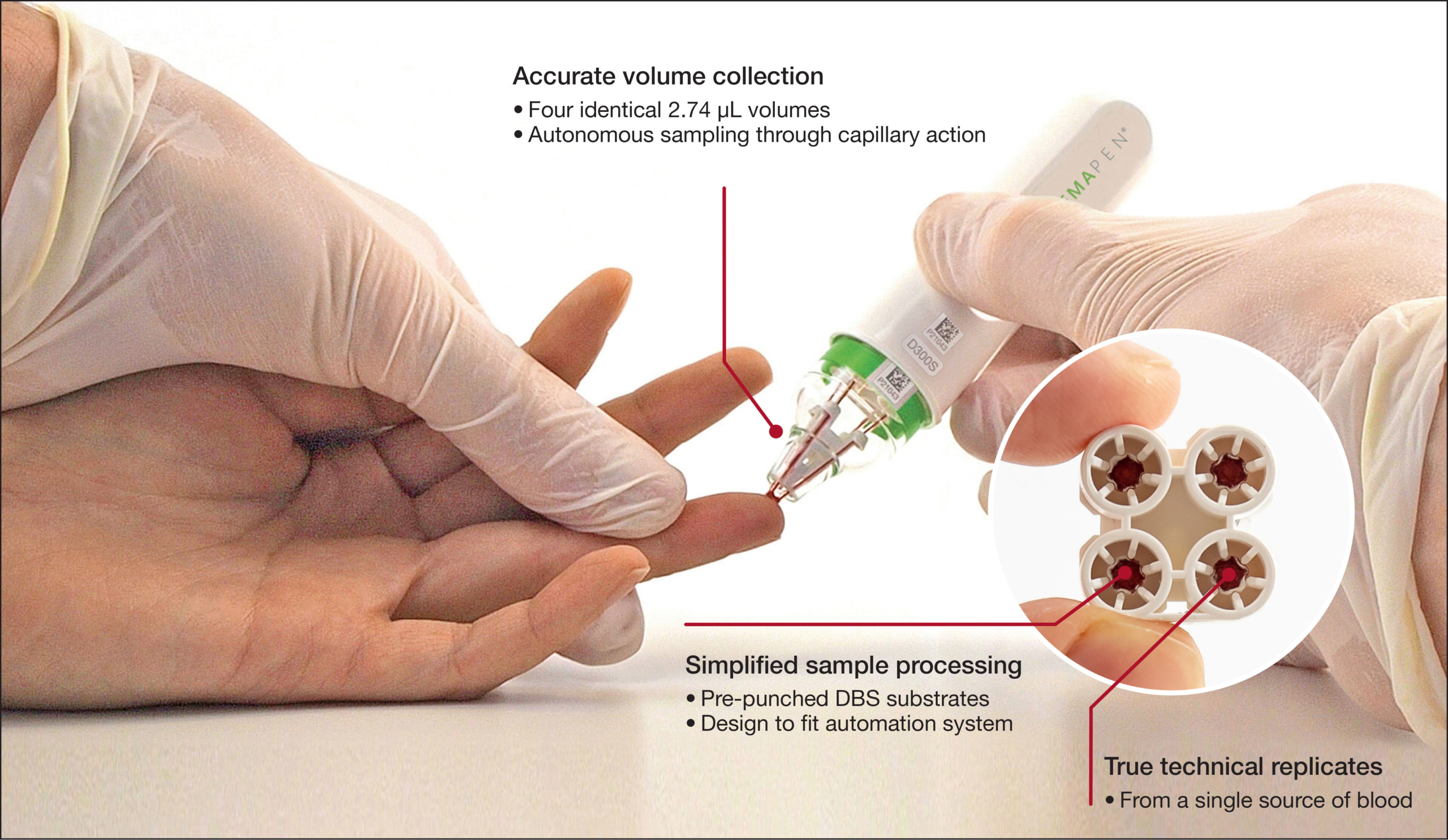
The hemaPEN® (Trajan Scientific and Medical, Melbourne, Australia) is one such patient-centric device that allows rapid and quality remote-sampling (Fig. 2). lt collects four samples from any single source, rapidly and with minimal volume. lts capillary-based technology ensures accurate and precise volume collection, while pre-punched DBS substrates store the samples inside a contained chamber.
Material and methods
A bioanalytical method for the quantification of TAC on volumetric DBS discs using liquid chromatography, positive electrospray ionization mode (ESI+), and coupled with tandem mass spectrometry (LC-ESI-MS/MS) was developed and validated according to the guidelines of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) and European Medicines Agency (EMA) [6, 7].
Both the hemaPEN and hemaPEN®-QC – a laboratory-centric version of the hemaPEN – were used to collect and store the blood samples. Each hemaPEN provided four DBS discs, each disc containing a volume of 2.74μL of blood and located inside a plastic DBS cartridge.
TAC analysis was performed using Shimadzu’s CLAM-2030, a fully automatic LC-MS preparation module connected to the LCMS-8060 LC-MS system.
All automated workflow and MS parameters were first optimized and set before validating the extraction method.
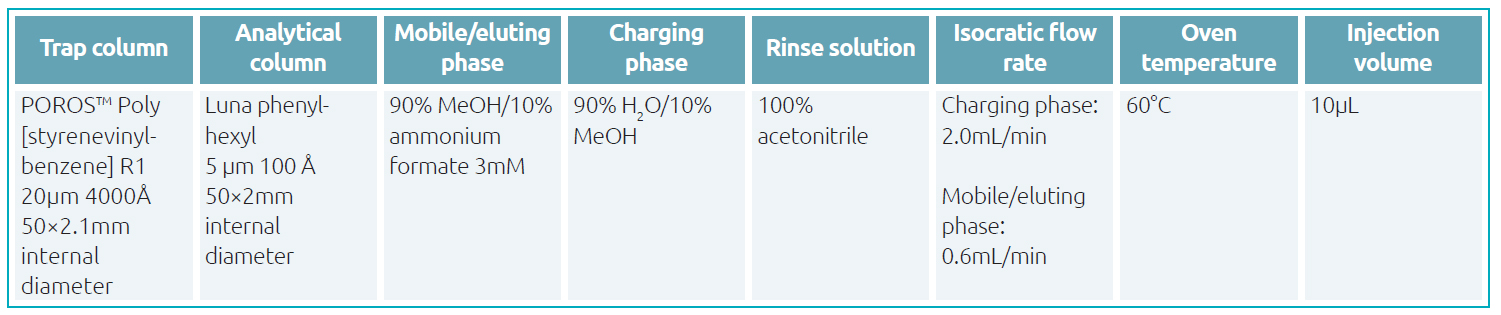
The treated samples were trapped on an online-SPE column using an isocratic charging phase and then separated at 60°C with an isocratic mode on an analytical column in 1.54min. Conditions were chosen for more Gaussian and intenser peaks (Table 1), allowing a lower limit of quantification of 1μg/L. The calibration ranged from 1 to 100μg/L.
Acquisition was performed in positive ionisation multiple-reaction monitoring (MRM) mode using LC-MS/MS, and quantification was done using stable isotope-labelled internal standards (13C-D2 TAC).
The validated parameters were: intra- and inter-assay, accuracy and precision, stability at different storage conditions, dilution integrity, selectivity, recovery and carry-over. Matrix and hematocrit effects were evaluated in a parallel manual sample preparation, as they do not depend on the preparation method but on the matrix itself.
Sample preparation
Collection of pre-spiked WB was performed using the hemaPENQC and left to dry for 2h minimum before processing. Patientcentric hemaPENs were used to validate the stability parameters.
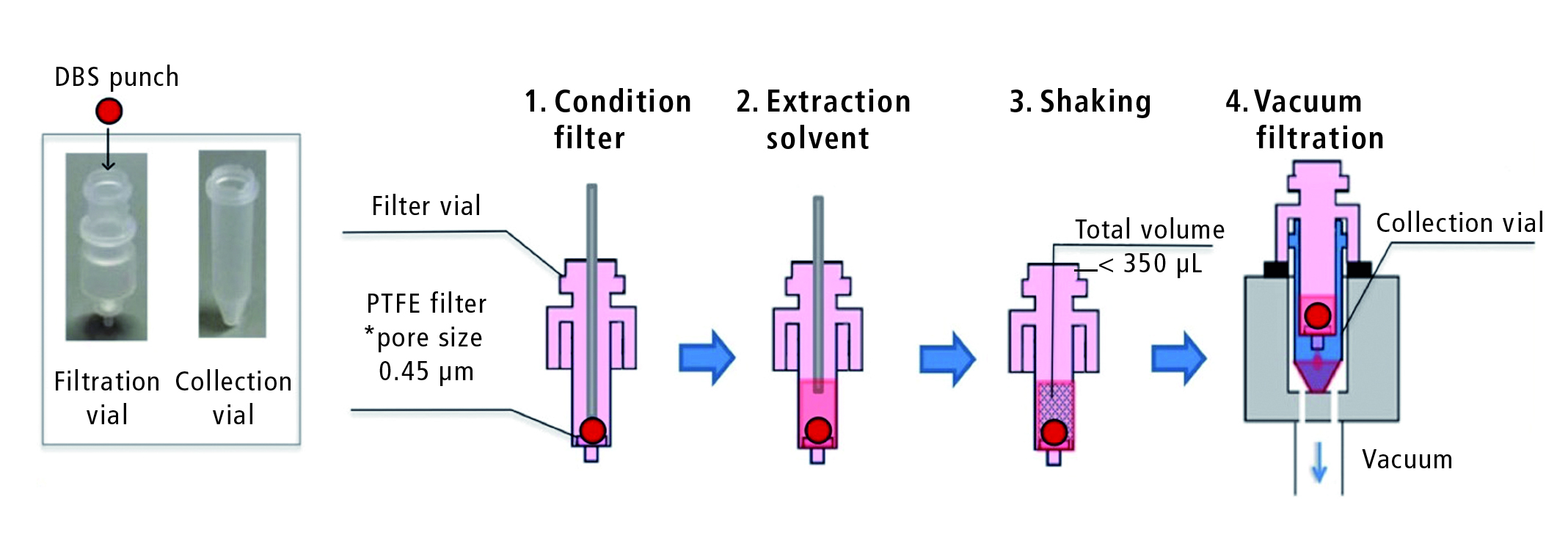
For each sample, one DBS disc was pushed out manually from the hemaPEN DBS cartridge into the filtration vial, itself placed over a dedicated collection vial (Fig. 3). Sequential automated sample preparation and injection then proceeded: briefly, 20μL of MeOH was added to condition the filtration vial’s filter, and 75μL of extraction solvent MeOH/H20 (80%/20%) with internal standard at 2.5mg/L was further added. The vial was then shaken for 120s at 2000rpm prior to 60s vacuum filtration (Fig. 3). The CLAM’s automated arm discarded the filtration vial and moved the collection vial to the liquid chromatography’s injector.
Results
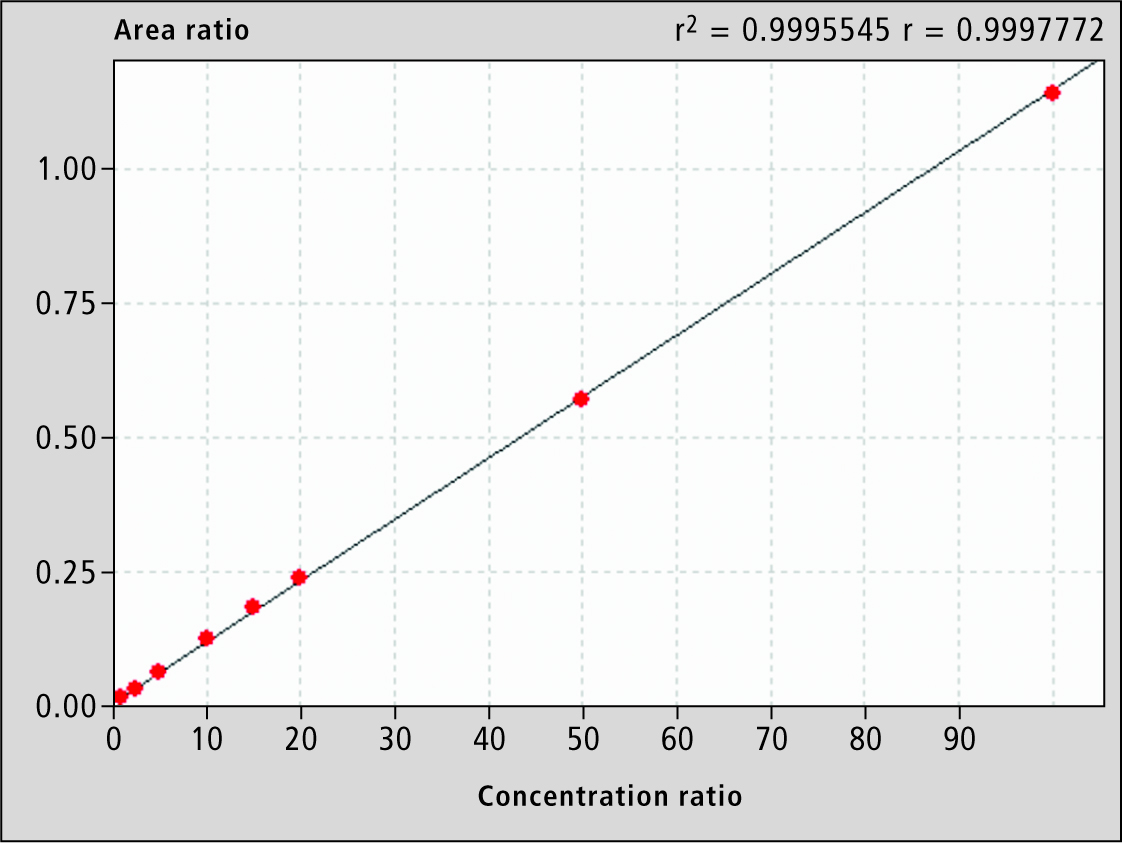
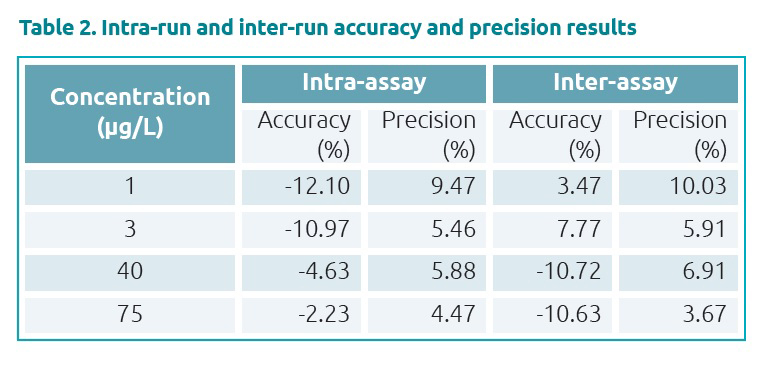
The assay was linear from 1 to 100μg/L (Fig. 4), with regression coefficients (r2) >0.998. lntra-assay and inter-assay imprecision and biases were all less than 15% (20% for the limit of quantitation) (Table 2).
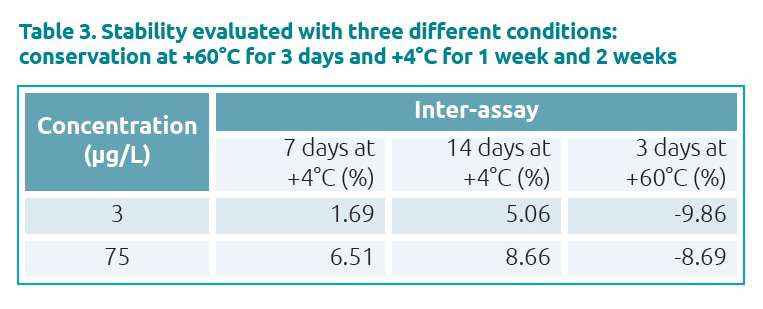
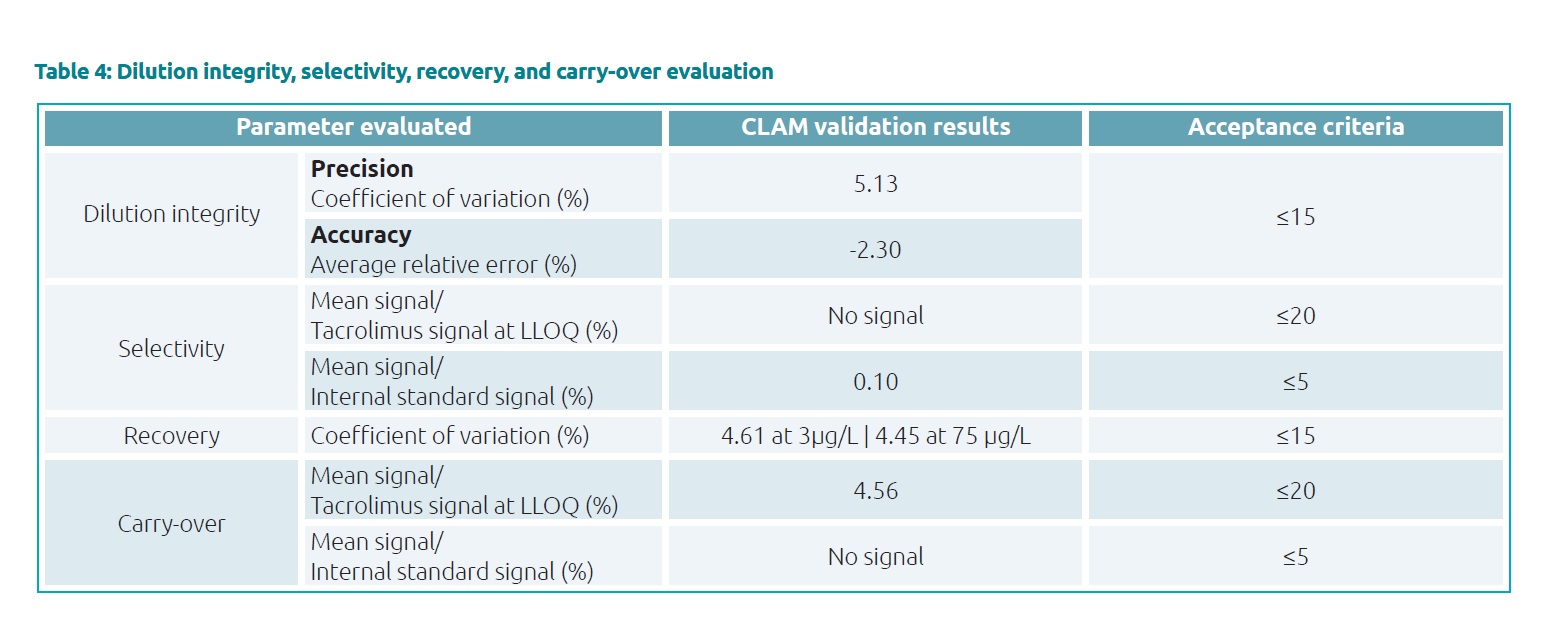
TAC on hemaPEN DBS samples was stable for at least 14 days at 4°C, and at least 72h at 60°C (Table 3). No carry-over was observed. Selectivity and dilution integrity were validated as well. The average recovery of TAC was 87.34% (Table 4).
No matrix effects were observed with manual preparation. Hematocrit had no effect on the results (data not shown).
Discussion
A reliable LC-MS/MS method has been developed for the measurement of TAC, sampled by hemaPEN, with an automated extraction on CLAM-2030.
Routine analytical laboratories could benefit from these automated processes of pre-treatment and LCMS analysis, leading to quicker availability of results for clinics and efficient resource allocation workflow for the laboratory. This approach is also associated with reduced operator errors and lower risk of infection during sample manipulation. A clinical validation project, led by an ethical committee, is going to clinically confirm the overall benefits of this workflow on transplant patients.
Disclaimer
hemaPEN® is a registered trade mark owned by Trajan Scientific Australia Pty Ltd. hemaPEN® is supplied for therapeutic or IVD use in Australia, New Zealand, UK, EU and USA only: ARTG number: 280007; CE mark, general IVD; US FDA number: D410490. Outside of the territories listed above, the hemaPEN is supplied for research purposes only and not for therapeutic or diagnostic use.
The author
Gauthier Rosé Department of Pharmacology and Toxicology,
Limoges University Hospital, France
E-mail: shimadzu@shimadzu.eu
References
1. Allison TL. Immunosuppressive Therapy in Transplantation. Nurs Clin North Am 2016; 51(1): 107–120.
2. Saint-Marcoux F, Woillard JB, Jurado C, Marquet P. Lessons from routine dose adjustment of tacrolimus in renal transplant patients based on global exposure. Ther Drug Monit 2013; 35(3): 322–327.
3. Wilhelm AJ, den Burger JC, Swart EL. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet. 2014; 53(11): 961–973 (https://link.springer.com/article/10.1007%2Fs40262-014-0177-7).
4. Robin T, Saint-Marcoux F, Toinon D, Tafzi N, Marquet P, El Balkhi S. Automatic quantification of uracil and dihydrouracil in plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2020; 1142: 122038.
5. Robin T, Barnes A, Dulaurent S, Loftus N, Baumgarten S, et al. Fully automated sample preparation procedure to measure drugs of abuse in plasma by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 2018 Aug;410(20):5071–5083.
6. Capiau S, Veenhof H, Koster RA, Bergqvist Y, Boettcher M, et al. Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology Guideline: development and validation of dried blood spot-based methods for therapeutic drug monitoring. Ther Drug Monit 2019; 41(4): 409–430.
7. Committee for Medicinal Products for Human Use. Bioanalytical method validation. European Medicines Agency 2015 (https://www.ema.europa.eu/en/bioanalytical-method-validation).
Acknowledgments
This study was a collaborative work between the following entities: Trajan Scientific and Medical, Shimadzu Europa GmbH and the Department of Pharmacology and Toxicology of the Limoges University Hospital. Analytical developments were performed by Gauthier Rosé as part of his Master’s degree, which took place at the Limoges University Hospital, with the help of NaÏma Tafzi (analytical engineer; Limoges) and Ryu Konoshita (Product specialist life sciences; Shimadzu). Dr Florian Lapierre (Trajan Scientific and Medical), Stéphane Moreau (Shimadzu Europa GmbH), Dr Caroline Monchaud and Prof. Franck Saint-Marcoux (Department of Pharmacology and Toxicology of the Limoges University Hospital) equally coordinated the whole study.