YKL-40: a new prognostic biomarker in patients with coronary artery disease
Inflammation is of importance for the progression of coronary artery disease. Until now, there has been no biomarker to monitor the effect of treatment regimes. YKL-40 is a new biomarker of inflammation, which if highly elevated in the disease, is a strong prognostic predictor of death and potentially can be used to monitor disease activity.
by Prof. J. Kastrup, Dr M. Harutyunyan-Bønsager and Dr N. D. Mygind
Clinical background
The number of patients with coronary artery disease (CAD) is increasing worldwide, and CAD is the most common cause of death in western countries. Although the prognosis and quality of life for patients has improved due to more aggressive and invasive treatment regimes, in the US someone will have a coronary event approximately every 25 seconds, and someone will die of one approximately every minute. Therefore CAD is an increasing economic burden and the total estimated direct and indirect costs of CAD in the US in 2010 were $503.2 billion [1].
Currently, there is a lack of new biomarkers for monitoring the effect of the patients’ treatment and for predicting their risk of a heart attack, heart failure and cardiac death.
Coronary artery disease and inflammation
It has been well established that inflammation plays an important role in development and progression of atherosclerosis in the coronary arteries [2]. Moreover, inflammation is also involved in the inflammatory pathways inducing extracellular matrix remodelling and heart failure progression [3]. The inflammatory biomarker high-sensitivity C-reactive protein (hs-CRP) is associated with atherosclerosis and the incidence of coronary events [4], but its association with the extent and severity of atherosclerosis remains controversial. Therefore, it is not very useful for continuous monitoring of treatment effects and progression of the disease.
The inflammatory biomarker YKL-40
YKL-40 is a glycoprotein mainly produced by macrophages and neutrophils, which are important for the development of atherosclerosis, and is stimulated by hypoxia [5]. Serum YKL-40 is suggested to be a biomarker of diseases characterized by inflammation [5] and its plasma concentration has been shown to increase reversibly in patients by more than 25% following an inflammatory stimulus.
YKL-40 is not a disease specific biomarker, but plays a role in cell migration and adhesion, angiogenesis, remodelling of the extracellular matrix, cell proliferation and differentiation [5]. Macrophages in atherosclerotic plaques, especially those located more deeply in the atherosclerotic lesion, express YKL-40 [6], and macrophages in early atherosclerotic lesions express the highest amount of YKL-40 mRNA. As Hs-CRP is mainly produced in the liver, it is likely that biomarkers such as YKL-40 (secreted from inflammatory cells within the atherosclerotic plaque) could be superior for monitoring CAD.
YKL-40 in healthy subjects
The normal YKL-40 value in a healthy subject from the general population has recently been published [7]. In 3130 subjects the median YKL-40 value was 40 µg/L and increased exponentially with age.
YKL-40 in coronary artery disease
Serum YKL-40 has been found to be increased in both acute and coronary artery disease [8]. Serum YKL-40 levels were also significantly increased in patients with acute ST-elevation myocardial infarction and thereafter consistently decreased from a maximum value just after the myocardial infarction and during a 360 day follow-up period towards its normal levels. Plasma YKL-40 levels were found to correlate inversely with left ventricular ejection fraction (LVEF) recovery, but not with infarct size in patients with STEMI [9, 10].
Although highly increased in patients with stable CAD, it has not been possible to detect any relationship between serum YKL-40 level and the degree of CAD as evaluated by the number of vessels involved or the degree of artery stenosis [11]. In patients with stable CAD, revascularization with balloon angioplasty of significant stable coronary artery lesions has no effect on YKL-40 levels within a 6 month follow-up period (unpublished data).
This indicates that YKL-40 not is a measurement of the amount of ischemia within the myocardium. Serum YKL-40 seems to be more a measurement of ongoing inflammatory activity rather than the presence of stabilized chronic lesions.
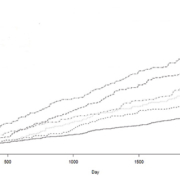
Therefore, it is very interesting that serum YKL-40 was a very strong prognostic biomarker for death within a 2.6 and 6 year follow-up period in patients with stable CAD [12, 13] [Fig. 1].
YKL-40 and heart failure
The consequence of CAD is often the development of severe heart failure. It has recently been demonstrated that serum YKL-40 is increased in heart failure and that YKL-40 is an independent significant prognostic biomarker for death [15]. It is interesting that serum YKL-40 measured in all-comers at acute hospital admission is a very strong predictor of death, especially within the first year, in patients with heart disease [16]. Of patients admitted with disease of the heart, those with elevated YKL-40 had a hazard ratio of death within the first year after discharge from the hospital at 2.5 compared to heart patients with normal serum YKL-40 levels. YKL-40 remained an independent biomarker of mortality, even after adjusting for other known risk factors such as age, hs-CRP and NT-proBNP [16].
YKL-40 for monitoring CAD activity
Statin treatment is used in CAD for lowering cholesterol levels. However, it also has an anti-inflammatory action. Therefore, it is very interesting that serum YKL-40 is significantly lower in patients with stable CAD on statin treatment compared to patients without [14] [Fig. 2].
This difference seems to be independent of the effect that statins have on lowering cholesterol levels, indicating that the YKL-40 level can be regulated by the direct anti-inflammatory action of statins [14]. This is unlike the situation with the inflammatory biomarker hs-CRP, which has been shown to correlate to cholesterol levels in statin-treated CAD patients [14].
Moreover, the mortality is also lower in stable CAD on statins compared to non-statins [12, 13]. This indicates that YKL-40 could be used to monitor the anti-inflammatory effect of statin treatment. Whether YKL-40 is also useful for
monitoring the effects of other anti-angina medications remains to be investigated.
Conclusion and future perspective
YKL-40 is a new inflammatory biomarker in ischemic heart disease. It is increased in both acute and chronic coronary artery disease and is a very strong diagnostic biomarker for death. It is suggested to be a mirror of the active inflammatory atherosclerotic processes in CAD, more than a measurement of degree of myocardial ischemia induced by stable coronary lesions. Since YKL-40 is lower in patients on statin treatment, it can potentially be used to monitor disease activity and the effect of anti-inflammatory or stabilizing treatment regimes.
Conflict of interest
A patent application (WO 2009/092382) is published and pending.
References
1. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Circulation 2012; 125(1): e2–e220.
2. Hansson GK. J Thromb Haemost 2009; 7 Suppl 1: 328–331.
3. Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, et al. J Card Fail 2008; 14(6): 467–474.
4. Corrado E, Rizzo M, Coppola G, Fattouch K, Novo G, Marturana I, et al. J Atheroscler Thromb 2010; 17(1): 1–11.
5. Kastrup J. Immunobiology 2012; 217(5): 483–491.
6. Boot RG, van Achterberg TA, van Aken BE, Renkema GH, Jacobs MJ, Aerts JM, et al. Arterioscler Thromb Vasc Biol 1999; 19(3): 687–694.
7. Bojesen SE, Johansen JS, Nordestgaard BG. Clin Chim Acta 2011; 412: 709–712.
8. Wang Y, Ripa RS, Johansen JS, Gabrielsen A, Steinbruchel DA, Friis T, et al. Scand Cardiovasc J 2008; 42(5): 295–302.
9. Nojgaard C, Host NB, Christensen IJ, Poulsen SH, Egstrup K, Price PA, et al. Coron Artery Dis 2008; 19(4): 257–263.
10. Hedegaard A, Ripa RS, Johansen JS, Jorgensen E, Kastrup J. Scand J Clin Lab Invest 2010; 70(2): 80–86.
11. Mathiasen AB, Harutyunyan MJ, Jorgensen E, Helqvist S, Ripa R, Gotze JP, et al. Scand J Clin Lab Invest 2011; 71(5): 439–447.
12. Kastrup J, Johansen JS, Winkel P, Hansen JF, Hildebrandt P, Jensen GB, et al. Eur Heart J 2009; 30(9): 1066–1072.
13. Harutyunyan M, Gotze JP, Winkel P, Johansen JS, Hansen JF, Jensen GB, Hilden J, Kjøller E, Kolmos HJ, Gluud C, Kastrup J. Immunobiology 2013; 218(7): 945–951.
14. Mygind ND, Harutyunyan MJ, Mathiasen AB, Ripa RS, Thune JJ, Gotze JP, et al. Inflamm Res 2011; 60(3): 281–287.
15. Harutyunyan M, Christiansen M, Johansen JS, Køber L, Torp-Petersen C, Kastrup J. Immunobiology. 2012; 217(6): 652–656.
16. Mygind ND, Iversen K, Køber L, Goetze JP, Nielsen H, Boesgaard S, Bay M, Johansen JS, Nielsen OW, Kirk V, Kastrup J. J Intern Med 2013; 273(2): 205–216.
The authors
Jens Kastrup* MD, DMSc; Marina Harutyunyan-Bønsager MD; and Naja Dam Mygind MD
Department of Cardiology B, The Heart Centre, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark
*Corresponding author
E-mail: jens.kastrup@regionh.dk