Zinc oxide nanorod-based acute kidney injury biomarker detection technology and potential clinical implications
We developed an ultrasensitive bioassay using micropatterned zinc oxide nanorods (ZnO NRs) for the multiplexed detection and quantification of trace levels of cytokines implicated in acute kidney injury (AKI) directly from patient samples. The remarkable limits of detection of the novel ZnO NR-based assay are compared directly with conventional methods.
by Manpreet Singh, Anginelle Alabanza, Lorelis E. Gonzalez, Weiwei Wang, Prof. W. Brian Reeves, and Prof. Jong-in Hahm
Introduction
Cytokines and chemokines are important immunoregulatory molecules produced by many cells such as neutrophils, monocytes, macrophages and T-cells that can serve as biomarkers of inflammatory diseases to predict and track disease pathogenesis [1–4]. Various cytokines and chemokines like interleukins (ILs) and tumour necrosis factors (TNFs) can serve as valuable clinical biomarkers of acute kidney injury (AKI), a rapidly acquired disorder associated with high morbidity and mortality that is commonly seen in hospitalized patients. As elevated levels of cytokines may reveal the activation of signalling pathways leading to inflammation and disease progression, methods enabling prompt and sensitive detection and quantification of multiple cytokines/chemokines simultaneously in a clinical setting are highly sought. Although conventional techniques such as enzyme-linked immunosorbent assays (ELISAs) are widely available and reliable, their applications may not be suitable for the rapid, multiplexed detection of weakly expressed cytokines due to their detection limits (DLs) of typically greater than tens of pg/mL, long assay times of several hours, and extensive serial workflows for detecting multiple protein analytes.
As the typical levels of important AKI-implicated cytokines and chemokines in healthy populations can often be well below the customary DLs of standard cytokine assays, which are generally around tens of pg/mL, there is great clinical interest in reducing the lower limits of detection down to the fg/mL range. In particular, the increasing need for early diagnosis and treatment in AKI and other cytokine-implicated diseases has driven the development of innovative detection schemes capable of reaching even lower DLs than have conventionally been offered. In this context, we have shown that zinc oxide nanorods (ZnO NRs) permit enhanced detection of fluorescence signals emitted by fluorophore-coupled biomolecules in the forms of custom-prepared oligonucleotide constructs and highly purified single-composition proteins in simple media [5–8]. In our most recent work, which is highlighted here, we have developed and validated an ultrasensitive fluorescence-based bioassay using micropatterned ZnO NRs as a novel optical platform for the multiplexed detection and quantification of two urinary biomarkers of AKI, tumour necrosis factor-α (TNF-α) and interleukin-8 (IL-8), in samples of patients at risk for and diagnosed with AKI [9].
In addition to the biomedical relevance of TNF-α and IL-8 in the pathophysiology of AKI, the biomarkers are ideal model cytokines and chemokines for this study due to the differences in their typical concentration levels found in human urine. The baseline expression of IL-8 in healthy populations generally ranges from tens of pg/mL to ng/mL and can be ascertained using conventional approaches, whereas TNF-α levels are typically below the DLs of traditional cytokine detection platforms. Accordingly, we performed ELISA- and ZnO NR-based assays on the same set of patient samples to first examine whether the highly expressed IL-8 levels agree between both detection methods and further demonstrated the detection capability of the ZnO NRs to reveal the ultralow protein levels of TNF-α that cannot otherwise be ascertained via ELISA.
Results and discussion
Overall approach of ZnO NRs-based fluorescence assay
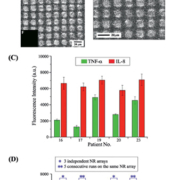
Using a micropatterned array of densely grown, vertically oriented ZnO NRs synthesized using a facile, low-cost, chemical vapour-phase method, we employed a sandwich assay scheme for the multiplexed detection of both AKI-relevant biomarkers. The sequential assay steps included incubation of the ZnO NR platform with primary TNF-α and IL-8 antibodies, bovine serum albumin for surface blocking, standards for both proteins for generating calibration curves or patient urine samples for determining biomarker levels in subject individuals, and fluorophore-conjugated secondary antibodies. In Figure 1(A), representative emission data from the multiplexed assay are qualitatively presented for Alexa 488-labelled TNF-α (left) and Alexa 546-labelled IL-8 (right). As a direct comparison, the panels in Figure 1(B) display scanning electron microscope (SEM) images of the ZnO NR array platform to show the morphology and dimensions of the individual square patches of ZnO NRs. The highly crystalline ZnO NRs do not exhibit any background fluorescence, as evidenced in the inset (F) of image 1(B), and, hence, all optical signals detected for protein quantification are derived only from the surface-adsorbed fluorophore-tagged biomolecules.
Reproducibility and calibration
In Figure 1(C), exemplar fluorescence intensity plots show the different amounts of TNF-α and IL-8 simultaneously detected from selected patient urine samples as obtained by averaging the optical signal from about 550 NR square patches on different areas of the same ZnO NR detection platform. The reproducibility of the fluorescence signal on the ZnO NRs-based platform is shown in Figure 1(D) in which the same patient sample was assayed five times on the same ZnO NR platform for intra-assay variability (**) as well as on three different ZnO NR plates for inter-assay variations (*) that may arise from assay or array-to-array differences. This scheme was conducted for two patient samples, and the coefficients of variation for the intra-assay (16.5% for TNF-α and 2.5% for IL-8) and inter-assay (12% for TNF-α and 2.8% for IL-8) results were found to be below the generally accepted value of 10–20%.
In order to quantitatively compare the levels of TNF-α and IL-8 obtained via the ELISA- and ZnO NRs-based assays, calibration curves were generated using standard solutions of each cytokine. The DLs of the ELISA-based method, defined as 2 standard deviations above the mean of 20 zero concentration replicates, were determined as 5.5 and 7.5 pg/mL for TNF-α and IL-8, respectively. On the other hand, the DLs of the ZnO NR platform, assessed using the upper boundary of blank samples with a 95% accuracy goal, were found to be 4.2 and 5.5 fg/mL for TNF-α and IL-8, respectively. The unparalleled sensitivity down to the several fg/mL range enabled by the ZnO NR platform can reveal the levels of weakly expressed, disease-implicated cytokines such as TNF-α to promote early clinical diagnostics.
IL-8 testing and statistical analysis
Following calibration, the same patient samples were assayed on both the ELISA and ZnO NR platforms for quantitative comparison between both assays. When comparing the highly expressed IL-8 levels in the urine of 38 patients that ranged between several tens of pg/mL to a few ng/mL, the ZnO NRs-based assay had strong statistical agreement with the ELISA-based results allowing for direct validation of the novel bioassay. In Figure 2(A), a correlative plot displays the IL-8 readings from the same patients determined by the ELISA and ZnO NR assays on the x and y axes, respectively. The linear fit of the data points, shown in the dashed red line, lies very close to the superimposed line of y = x, shown in black, indicating excellent agreement between the two assay methods. In Figure 2(B), a histogram distribution chart reporting the differences in IL-8 readings between the two methods shows the majority of IL-8 readings from both assays fell within the range of ±2.5 pg/mL of each other. The IL-8 levels were further evaluated using the Bland-Altman analysis in Figure 2(C & D) in which the differences between the ELISA and ZnO NR readings for each patient were plotted against the mean concentration values. As shown, the data analysed over a large range of concentrations centred near the black lines, which represent the case of equivalent IL-8 readings obtained from the two different assays. The results of these comparative analyses validate the ZnO NR platform as a reliable technique to accurately quantify urinary biomarker proteins directly from patient samples.
TNF-α testing
To substantiate the applicability of the ZnO NR platforms in ultrasensitive cytokine detection using the weakly expressed biomarker of TNF-α, the protein levels for 46 patients were determined using both assay platforms. As seen in Figure 3(A), many of the patient samples exhibited values too close or below the DL of the ELISA assay (5.5 pg/mL) and are marked accordingly as grey blocks in the ELISA row. By contrast, the TNF-α values of all the patients were successfully quantified on the ZnO NR platform, well below several tens of pg/mL and into the low fg/mLrange. For the ZnO NR row in Figure 3(A), those samples that could not be measured via ELISA are shown with a magnifier sign, and their TNF-α concentrations, as determined by the ZnO NRs-based assay, are then shown in Figure 3(B & C) on two different scales for clarity. As demonstrated, the optical signal enhancement provided by the ZnO NR array platform enables the ultrasensitive detection of trace levels of proteins directly from patient samples.
Advantages of the ZnO NR-based approach and future outlook
Within the realm of biodetection, the ZnO NR-based approach can provide many direct advantages including facile platform fabrication, desirable optical properties, biocompatibility, and promising multiplexed/high-throughput integration capacities. The ZnO NR arrays are easily fabricated using a gas-phase method through well-established synthesis procedures and can be used directly after growth without any post-synthetic modifications. Further, the highly crystalline NRs exhibit many desirable optical properties including no intrinsic fluorescence (i.e. absence of autofluorescence) as well as enhancement of the optical intensity and photostability of nearby signal emitters. Since the ZnO NRs do not display any photoluminescence in the visible and near-infrared range, they do not interfere with the spectroscopic profiles of fluorophores commonly used in biology and biomedical detection. At the same time, the reduced dimensions and high shape anisotropy of the ZnO NRs enable optical enhancement and prolonged stability of the signal from fluorophore-tagged biomolecules adsorbed on their surface allowing for the ultrasensitive detection of trace levels of bioconstituents.
In addition to the demonstrated sensitivity permitted by the ZnO NR-based platform, the cytokine bioassay also has the direct advantages of rapid analysis, minimal volume requirements, and reusability. The multiplexed detection was achieved with 90 min of total assay time and only 60 μL of total bioreagent/sample volume using commonly employed fluorescence microscopy instrumentation. Further, the highly biocompatible ZnO NRs platform was found to withstand at least 25 repeated assays in complex biological and chemical reaction environments that include urine samples.
As modern automation strategies in high-throughput screening have seen great advancements in the sophistication of robotic sample delivery strategies and the detection of many analytes simultaneously via multichannel optical sensors, the ZnO NR-based platform may be able to provide much-sought detection sensitivity when integrated into these breakthrough technologies. In the microarray, each square patch of densely grown ZnO NRs with a typical dimension of 3~50 μm in side length can be configured to serve as a discrete detection element for different patient samples when coupled with appropriate sample delivery and multiplexed optical sensing/readout platforms. The demonstrated detection capabilities combined with this integration potential suggests that the ZnO NR-based approach serves as more than just an alternative or tandem detection platform to existing methods, but rather provides an advanced approach which allows the much needed, ultrasensitive detection of biomarker proteins in samples that exhibit concentration levels much lower than those which standard techniques can ascertain.
Conclusion
We successfully demonstrated a ZnO NRs-based fluorescence bioassay for the rapid, ultrasensitive, quantitative and multiplexed detection of AKI-related biomarkers in patient urine samples. We first statistically validated the ZnO NR-based approach against a conventional ELISA-based method by comparing the measurements of highly expressed levels of IL-8 that were above the DLs of ELISA. We further revealed the full detection capabilities of the ZnO NRs platform by quantifying ultratrace amounts of a weakly expressed cytokine, TNF-α, whose levels in urine are often below the DLs of conventional cytokine assays. The unparalleled detection sensitivity and other discussed advantages of the ZnO NR-based bioassay can be readily extended to advance other optical-sensing applications in biological research and clinical diagnostics.
Acknowledgement
This article is a summary of the work first presented in Singh M, Alabanza A, Gonzalez LE, Wang W, Reeves WB, Hahm J. Ultratrace level determination and quantitative analysis of kidney injury biomarkers in patient samples attained by zinc oxide nanorods. Nanoscale 2016; 8: 4613–4622 [9].
References
1. Feldmann MJ. Many cytokines are very useful therapeutic targets in disease. Clin Invest. 2008; 118: 3533–3536.
2. Fichorova RN, Richardson-Harman N, Alfano M, et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem. 2008; 80: 4741–4751.
3. Borish LC, Steinke JWJ. Cytokines and chemokines. Allergy Clin Immunol. 2003; 111: S460–S475.
4. Nathan C, Sporn M. Cytokines in context. J Cell Biol. 1991; 113: 981–986.
5. Adalsteinsson V, Parajuli O, Kepics S, et al. Ultrasensitive detection of cytokines enabled by nanoscale ZnO arrays. Anal Chem. 2008; 80: 6594–6601.
6. Dorfman A, Kumar N, Hahm J. Nanoscale ZnO-enhanced fluorescence detection of protein interactions. Adv. Mater. 2006; 18: 2685–2690.
7. Singh M, Song S, Hahm J. Unique temporal and spatial biomolecular emission profile on individual zinc oxide nanorods. Nanoscale 2014; 6: 308–315.
8. Singh M, Jiang R, Coia H, et al. Insight into factors affecting the presence, degree, and temporal stability of fluorescence intensification on ZnO nanorod ends. Nanoscale 2015; 7: 1424–1436.
9. Singh M, Alabanza A, Gonzalez LE, et al. Ultratrace level determination and quantitative analysis of kidney injury biomarkers in patient samples attained by zinc oxide nanorods. Nanoscale 2016; 8: 4613–4622.
The authors
Manpreet Singh1 BS, Anginelle Alabanza1 BS, Lorelis E. Gonzalez1 BS, Weiwei Wang2 BS, W. Brian Reeves3 MD, and Jong-in Hahm*1 PhD
1Department of Chemistry, Georgetown University, Washington, DC 20057, USA
2Division of Nephrology, The Penn State College of Medicine, Milton S. Hershey Medical Center, Hershey, Pennsylvania 17033, USA
3Department of Medicine, University of Texas Health Sciences Center at San Antonio, San Antonio, TX 78229, USA
*Corresponding author
E-mail: jh583@georgetown.edu