Direct thrombin inhibitor assays
We have investigated the effects of three (Lepirudin, Argatroban and Bivalirudin) direct thrombin inhibitors (DTI) on routine and dedicated assays.
We found routine tests to be non-discriminative between concentrations of different DTI. The dedicated Hemoclot assay showed identical lineair increases for all three DTI.
We conclude that a dedicated calibrated assay based on a diluted thrombin time (Hemoclot) appears to be the most suitable assay for monitoring purpose.
by Dr Joyce Curvers, Dr Volkher Scharnhorst and Dr Daan van de Kerkhof
Clinical background
The use of direct thrombin inhibitors (DTIs) for prophylactic or therapeutic anticoagulation is increasing due to their predictable bioavailability, short half life and limited interaction with other medication [1-5]. The current idea is that the newer anticoagulants should not require laboratory monitoring because of these advantages. However, although monitoring of anticoagulant therapy may not be required for ‘standard’ patients, patients with an increased bleeding risk, specific co-medication (such as amiodarone or bridging therapy with coumarins), or a deviant body mass or water homeostasis (e.g. neonates, during pregnancy, the obese, the elderly, in renal insufficiency, oedema, cardiac disease) may still require occasional blood analysis. In addition when the compliance or effectiveness of the anticoagulants is doubted, measurement of the coagulation status can be crucial for the correct treatment of a patient. Since DTIs interfere with the central clotting enzyme thrombin, almost every coagulation assay is affected by its presence in blood. This also accounts for routinely used assays such as the aPTT or PT (and INR) [6].
Up to date, there is no consensus on how oral or intravenous administrable DTI should be monitored and specifically which assay should ideally be used [6,7]. In this study we performed an in vitro study in which we investigated the effect of increasing concentration levels of three DTIs: lepirudin, bivalirudin and argatroban in six plasma pools on aPTT, PT, TT and on dedicated DTI-assays (Hemoclot from Hyphen BioMed and Ecarin Clotting Time from STAGO) on a coagulation analyser (STA-R Evolution, Roche).
Materials and methods
Six different pools (N>20 samples per pool) were collected from residual plasma from patients with aPTT and PT values within reference limits (assuming that patients did not take any anticoagulant medication based on their normal aPTT and PT values).
Argatroban (Arganova, Mitsubishi Pharma, lot PF41977, 100 mg/mL) and lepirudin (Refludan, Pharmion, lot 24661611L, 50mg) were provided by the local hospital pharmacy. Bivalirudin (Angiox or angiomax, The Medicines company, lot 1574697, 250 mg) was a kind gift from the Medicines Company. All DTIs were diluted with saline (0,9% NaCl) to 5 g/L. These stock solutions were spiked into the pooled plasmas (N=6) to reach final concentrations of 1, 2, 3, 4 and 5 mg/L. Therapeutic doses of DTI are currently advised at 2 mg/L (according to package leaflet). Different plasma pools with each different concentration of different DTIs were frozen in triplicates at <-70˚C until time of measurement.
Clotting times in the aPTT, prothrombin time (PT) and thrombin time (TT) as well as the dedicated assays Hemoclot (a diluted TT) and the Ecarin Clotting Time (ECT) were recorded.
Results
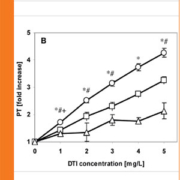
For all thrombin inhibitors investigated here, the fold increase compared to no DTI in six pools measured in routine tests (aPTT, PT and thrombin time) are shown in Figure 1. The aPTT shows a non-linear concentration-response relationship with a more gradual increase at higher DTI concentrations resulting in a limited sensitivity of the assay in this range. The concentration-response relationship for the PT was linear but with different sensitivities for the different DTIs. The low sensitivity was found especially for bivalirudin and lepuridin with respectively a maximum 2- and 3-fold increase in PT coagulation time at 5 mg/L. The thrombin time also showed a linear concentration-response relationship, with a high increase in coagulation time as function of concentration, especially for lepuridin, exceeding the maximum installed measuring range (i.e. 240 sec) of the STA-R evolution.
Figure 2 shows the data for the dedicated thrombin inhibitor tests. Similar results as for the PT were observed for the ECT, also with respect to the differences between different direct thrombin inhibitors. Lepirudin showed an increase in ratio up to 5-fold baseline value in the ECT. The increase in the Hemoclot was linear for all DTIs with similar increase as a function of concentration measured.
Conclusion
Concluding, dedicated DTI assays overcome the drawbacks of routine assays such as the PT, aPTT or TT, in which the ability to discriminate between different concentrations is insufficient. This would suggest that monitoring DTIs using the aPTT is obsolete. We have shown that dose-response curves of DTIs in dedicated assays such as the Hemoclot and ECT are acceptable. Moreover, they can be applied in a routine setting, have short turn around times and can be used to distinguish inappropriate from appropriate dosing without the necessity of reanalysis after dilution. Given that a calibrator is included in the assay kit and the test gives similar result for different DTI formulations, the Hemoclot assay appears to be the most suitable assay for monitoring purposes (apparent in this study). As more new oral thrombin inhibitors such as dabigatran etexilate find their way into troutine practice, dedicated assays may aid the clinician in better decision making concerning anticoagulant therapy, especially in certain groups of patients in need of monitoring. However, research is needed to properly determine therapeutic and prophylactic concentration ranges, with calibrated dedicated DTI assays.
Current status
The administration of (oral and) intravenous direct thrombin inhibitors is increasing, since more applications are becoming available. The pharmaceutical companies pay little attention to the fact that, in certain situations, indication of the concentration is warranted.
We are currently validating a calibrated assay based on a diluted thrombin time for use in our laboratory (and clinic), as are several other laboratories nation-wide.
Future prospects
Up to now little is known about interference of different anticoagulants combined with DTI (e.g. during bridging therapy) and the effects on the different dedicated assays. Future research will show the value of the different DTI assays in monitoring patients in order to distinguish proper dosing from under dosage or over dosage.
Moreover, standardisation and calibration of (present and new) dedicated assays for the measurement of DTI is a major issue of concern. Therefore we are currently conducting research in which a comparison of coagulation assay results with actual concentrations of the different DTI (measured with LCMSMS) is investigated.
Notification
Part of this publication is included in a manuscript that will be published in the American Journal of Clinical Pathology.
References
1. Di Nisio M, Middeldorp S, Buller HR. Direct thrombin inhibitors. N Engl J Med 2005; 353: 1028-1040.
2. Stone GW, Witzenbichler B, Guagliumi G et al. HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008; 358: 2218-2230.
3. Mehran R, Lansky AJ, Witzenbichler B et al. HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374: 1149-1159.
4. Connolly SJ, Ezekowitz MD, Yusuf S et al. RE-LY steering committee and investigators Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139-1151. Erratum in: N Engl J Med 2010 Nov 4;363(19):1877
5. Schulman S, Kearon C, Kakkar AK et al. for the RE-COVER study group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361: 2342-2352.
6. Gosslin RC, Dager WE, King JH et al. Effect of direct thrombin inhibitors, bivalirudin, lepirudin and argatroban, on prothrombin time and INR values. Am J Clin Pathol 2004; 121: 593-599.
7. Van Ryn J, Stangier J, Haertter S et al. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103: 1116-1127.
The authors
Joyce Curvers PhD, Volkher Scharnhorst PhD and Daan van de Kerkhof PhD
Clinical Laboratory
Catharina Hospital Eindhoven
Eindhoven
The Netherlands