Galactosemia is an inborn error of metabolism caused by the deficiency of any of the three principal enzymes (GALT, GALK and GALE) involved in the Leloir pathway. The application of urinary galactitol as a diagnostic and monitoring marker for galactosemia has been extensively researched but the practice varies in different centres. The Willink Biochemical Genetic Laboratory has recently developed and evaluated a method to quantitate urinary galactitol by gas chromatography–mass spectrometry and revisited its use as a first-line diagnostic test for galactosemia. The analytical performance characteristics of the method, established age-related reference ranges, and the relationship between urinary galactitol excretion and hepatic dysfunctions will be discussed.
by Yuh Luan Choo, Teresa Hoi-Yee Wu, Jackie Till and Dr Mick Henderson
Galactosemia: an overview
Galactosemia is a group of three inborn errors of galactose metabolism each with an autosomal recessive inheritance pattern. The deficiency or absence of galactose-1-phosphate uridyltransferase (GALT), galactokinase (GALK) or galactose-4-epimerase (GALE) enzymes involved in the Leloir pathway leads to toxic accumulation of galactose, hence the term ‘galactosemia’. Classical galactosemia is the most common form of galactosemia caused by GALT deficiency. The prevalence of classical galactosemia varies greatly across different populations in the world, i.e. 1 : 10 000–1 : 20 000 live births in Ireland, 1 : 25 000-1 : 44 000 live births in the United Kingdom, 1 : 50 000 cases in the United States, 1 : 100 000 newborns in Japan, and relatively low frequency in Asian populations [1]. GALK deficiency has a high prevalence of 1 : 1600 in the Romani Gypsy population [2], but in other populations GALK and the GALE deficiency are more rare and can present with acute and life-threatening clinical signs and symptoms, typically manifested within the first few days to weeks of life after consumption of breast milk and galactose-containing formula. Clinical symptoms such as jaundice, vomiting, failure to thrive and poor feeding are commonly observed in galactosemic babies [3]. Signs and symptoms of abnormal carbohydrate metabolism, kidney and liver dysfunction including aminoaciduria, hepatomegaly, hypoglycemia and elevated blood galactose and urinary galactitol are characteristic of this disorder. Untreated galactosemia can potentially lead to neonatal death. Early diagnosis and treatment is critical and usually life-saving. However, there are long-term clinical complications, including cataracts, short stature, neurodevelopmental problems, premature ovarian failure, developmental delay and impaired cognitive functions [4].
Biochemical tests for galactosemia and their limitations
Newborn bloodspot screening (NBS) for galactosemia is not currently recommended by the United Kingdom Newborn Screening Committee because it fails to meet their strict criteria. Current tests have high false-positive rates and early treatment is only partially successful. However, galactosemia is frequently detected under the existing protocol owing to affected babies having elevated phenylalanine (≥200 µmol/L) and tyrosine (≥240 µmol/L) and so are investigated for probable liver diseases [5].
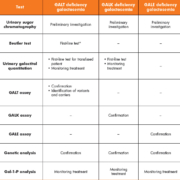
To date, a small number of laboratory tests are offered by specialist metabolic centres in the UK to aid the diagnosis and monitoring of galactosemia, including urinary sugar chromatography, the Beutler fluorescent spot test, urinary galactitol quantitation, quantitative assays of erythrocyte GALT, GALK and GALE enzymes, genetic analysis and galactose-1-phosphate (Gal-1-P) analysis (Table 1).
Urinary sugar chromatography
Increased urinary excretion of galactose, a feature of galactosemia, will give rise to a positive reducing substances result. The identification of the sugar is possible by a chromatography technique, as is the field method in the UK. These are useful first-line tests; however, false-negative results may be seen in patients who have already started a lactose-free diet.
Beutler test
Another commonly used first-line test that qualitatively detects the activity of GALT is the Beutler fluorescent spot test. This is a robust, technically simple test that works well in most situations. However, false-negative results could be expected following a blood transfusion. Also as the endogenous enzyme glucose-1-phosphate dehydrogenase (G6PD) is used as a linked enzyme in the Beutler method, G6PD deficiency will lead to a false-positive result.
GALT, GALK and GALE enzyme assay
The gold standard diagnostic tests are the quantitative assay for GALT, GALK and GALE to distinguish and confirm the three forms of galactosemia. However, blood transfusion will affect the validity of the enzyme results in the same manner as the Beutler test. Detection of the enzyme activities in lymphocytes may be helpful but all of these assays are laborious and time-consuming.
Galactose-1-phosphate (Gal-1-P) quantitation
The quantitative measurement of galactose-1-phosphate (Gal-1-P) is another technically complicated test that is useful to support the diagnosis in all forms of galactosemia. Gal-1-P has also been used as a biomarker to monitor dietary compliance in galactosemic patients; however, it is not a reliable marker for long-term monitoring because it reflects only the galactose ingestion in the past 24 hours and poorly correlates with long-term clinical outcome [6].
Urinary galactitol quantitation
Urinary galactitol, an end product of galactose formed by an alternative pathway, is invariably excreted in significant quantities in patients with all forms of galactosemia. As galactose is produced endogenously, the level of urinary galactitol is expected to be less affected by the dilutional effect of the blood transfusion or the exogenous/dietary source of galactose. In comparison to normal healthy controls, urinary galactitol excretion is significantly elevated at birth in all forms of galactosemia, including the milder phenotypes of GALT, i.e. S135L homozygosity [7] and in the Duarte variants [8]. The level of urinary galactitol decreases rapidly following commencement of dietary treatment but still remains above the reference ranges for normal healthy controls [7]. However, several studies have shown that galactitol is not correlated with dietary galactose intake or erythrocyte Gal-1-P concentration [8], nor with the development of long-term complications in patients with classical galactosemia [9]. In addition, the high intra-individual biological variability of urinary galactitol may limit its value in disease investigation and monitoring [10].
The practice in the diagnosis and monitoring of galactosemia varies widely, in particular on the use of urinary galactitol. The latest international guideline for classical galactosemia recommended that although urinary galactitol is unsuitable for disease monitoring, it could be used as a ‘supportive diagnostic test’ following blood transfusions [11], a treatment frequently used in neonatal care units. However, this test is not widely available and may be underused. Further research is necessary to evaluate the clinical usefulness of urinary galactitol in aiding the diagnosis and monitoring of galactosemia.
Measurement of urinary galactitol
Galactitol is the toxic metabolic by-product formed intracellularly following reduction of galactose by aldose reductase. Galactitol is subsequently excreted in the urine as it cannot be further oxidized by sorbitol dehydrogenase. This sugar alcohol has been extensively studied in urine, blood, amniotic fluid, liver, kidney, cardiac muscle, skeletal muscle, brain and the eye lens. Most clinically relevant data were derived from investigations on urinary galactitol. The analytical methods employed for identification and measurement of urinary galactitol have involved gas–liquid chromatography with trimethylsilyl (TMS) or methoxylamine-acetate derivatives, isotope dilution gas chromatography–mass spectrometry (GC-MS) with acetate derivative, reverse-phase high-performance liquid chromatography, thin-layer chromatography and proton magnetic resonance spectroscopy. Most research reported that GC-MS is particularly suitable for the quantitation of urinary polyols as it offers high resolution, great sensitivity and rapid analytical speed [12].
Urinary galactitol quantitation by gas chromatography–mass spectrometry
The Willink Biochemical Genetic Laboratory conducted a preliminary study on urinary galactitol quantitation by using a GC-MS method to evaluate the key analytical validation components, establish the age-related reference ranges, and to study the relationship between urinary galactitol excretion and hepatic dysfunctions. The study included plain urine samples from two known patients with galactosemia, random urine samples from eight unaffected patients with suspected hepatic dysfunction, and 120 individuals unaffected by galactosemia, received in the Willink Laboratory for a metabolic screen. The procedure was modified from the method described by Pettit et al. and Allen et al. based on the method principle of acetate derivatives formation followed by separation and detection using GC-MS [13, 14]. The method was linear from 2.5 µmol/L to 330 µmol/L. The lower limit of detection (LoD) and lower limit of quantification (LoQ) were 3 µmol/L and 9 µmol/L. Intra- and inter-assay precisions were 1.41–6.22% and 2.54–17.04% respectively at levels across the measuring range. We used a total of 27 samples from the ERNDIM (European Research Network for evaluation and improvement of screening, Diagnosis and treatment of Inherited Disorders of Metabolism) ‘Specialist Assays in Urine’ external quality assessment (EQA) scheme to test if our method was in agreement with those of other specialist laboratories. Figure 1 showed that the results from the GC-MS method were in good agreement with the method means (R2=0.944). We showed that samples for urinary galactitol measurement were stable up to 7 days under storage at −20 °C, 4 °C and room temperature. Our findings and other studies demonstrated that urinary galactitol excretion in both normal and galactosemic subjects are age-dependent, with the highest excretion at a younger age (Fig. 2). A minimal amount of galactitol can be found in urine samples of healthy individuals owing to the generation of galactose by endogenous metabolic reactions. Newborns are expected to excrete a greater amount of galactitol than older children as the neonatal liver is not yet fully developed and, thus, less effective in metabolizing the increased load of galactose after milk feeding. The age-related reference ranges were ≤85, ≤68, ≤29, ≤23, ≤9 and ≤4 µmol/mmol creatinine for the 0–3 months, 4–11 months, 1–2 years, 3–6 years, 7–15 years and >15 years age groups, respectively. In our study, galactosemic patients excreted 9-fold to ≥800-fold more urinary galactitol than the age-matched control group, whereas non-galactosemic patients with suspected hepatic dysfunction excreted 3-fold more. An elevated urinary galactitol result alone is does not identify whether galactosemia is caused by enzyme deficiency in the Leloir pathway or by other secondary causes. It is of utmost importance to consider further biochemical and radiological investigations for patients with hepatic dysfunctions and metabolic disorders in order to differentiate and confirm the diagnosis of hypergalactosemia.
Conclusion and future work
Further work is required for a comprehensive analytical and clinical validation of the test method, but our preliminary data are promising and demonstrate that the GC-MS quantitation of urinary galactitol would be acceptable for the diagnosis of galactosemia. Urinary galactitol is potentially very useful as a supportive diagnostic test following blood transfusions and its use should be encouraged. Its application as a first-line test for all forms of galactosemia is undisputable. A full evaluation of its clinical application will be possible following implementation of this assay into routine service in the Willink Biochemical Genetics Laboratory.
Acknowledgements
We would like to thank Graeme Smith and James Cooper for their technical expertise in helping to set up and validate the GC-MS assay for galactitol in our laboratory. We would also like to thank Ann Brown and the staff of the Clinical Chemistry Department at Southmead Hospital, Bristol, for sharing their in-house standard operating procedure for this method and demonstrating its use within their laboratory.
The Willink Laboratory acknowledges the use of data derived from ERNDIM EQA materials in this publication. The use of ERNDIM EQA materials does not imply that ERNDIM endorses the methods used or the scientific validity of the findings in this publication. ERNDIM (www.erndim.org) is an independent, not for profit foundation that provides EQA schemes in the field of inborn errors of metabolism with the aim of improving diagnosis, treatment and monitoring of inherited metabolic diseases.
References
1. Saleem U, Mahmood S, Kamran SH, Mutt MA, Ahmad B. Prevalence, epidemiology and clinical study of galactosemia. J App Pharm 2012; 4(1): 524–530.
2. Kalaydjieva L, Perez-Lezaun A, Angelicheva D, Onengut S, Dye D, Bosshard NU, Jordanova A, Savov A, Yanakiev P, et al. Founder mutation in the GK1 gene is responsible for galactokinase deficiency in Roma (gypsies). Am J Hum Genet 1999; 65(5): 1299–1307.
3. Waggoner DD, Buist NRM, Donnell GN. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis 1990; 13(6): 802–818.
4. Walter JH, Collins JE, Leonard JV, Chiswick M and Marcovitch H. Recommendations for the management of galactosaemia commentary. Arch Dis Child 1999; 80(1): 93–96.
5. UK National Screening Committee. Screening for galactosaemia: external review against programme appraisal criteria for the UK National Screening Committee (UK NSC). Bazian Ltd. 2014. http://legacy.screening.nhs.uk/screening-recommendations.php.
6. Van Calcar SC, Bernstein LE, Rohr FJ, Scaman CH, Yannicelli S, Berry GT. A re-evaluation of life-long severe galactose restriction for the nutrition management of classic galactosemia. Mol Genet Metab 2014; 112(3): 191–197.
7. Palmieri M, Mazur A Berry GT Ning C, Wehrli S, Yager C, Reynolds R, Singh R, Muralidharan K, et al. Urine and plasma galactitol in patients with galactose-1-phosphate uridyltransferase deficiency galactosaemia. Metabolism 1999; 48: 1294–1302.
8. Ficicioglu C, Hussa C, Gallagher PR, Thomas N, Yager C. Monitoring of biochemical status in children with Duarte galactosemia: utility of galactose, galactitol, galactonate, and galactose 1-phosphate. Clin Chem 2010; 56(7): 1177–1182.
9. Cleary MA, Heptinstall LE, Wraith JE, Walter JH. Galactosaemia: relationshiop of IQ to biochemical control and genotype. J Inherit Metab Dis 1995; 18, 151–152.
10. Hutcheson ACJ, Murdoch-Davis C, Green A, Preece MA, Allen J, Holton JB, Rylance G. Biochemical monitoring of treatment for galactosaemia: biological variability in metabolite concentrations. J Inherit Metab Dis 1999; 22(2): 139–148.
11. Welling L Bernstein LE, Berry GT, Burlina AB, Eyskens F, Gautschi M, Grünewald S, Gubbels CS, Knerr I, et al. International clinical guideline for the management of classical galactosaemia: diagnosis, treatment, and follow-up. J Inherit Metab Dis 2017; 40(2): 171–176.
12. Haga H, Nakajima T. Determination of polyol profiles in human urine by capillary gas chromatography. Biomed Chromatogr 1989; 3(2): 68–71.
13. Pettit BR, King GS, Blau K. The analysis of hexitols in biological fluid by selected ion monitoring. Biomed Mass Spectrom 1980; 7(7): 309–313.
14. Allen JT, Holton JB, Lennox AC, Hodges IC. Early morning urine galactitol levels in relation to galactose intake: A possible method of monitoring the diet in galactokinase deficiency. J Inherit Metab Dis 1988; 11(S2): 243–245.
The authors
Yuh Luan Choo1 MSc; Teresa Hoi-Yee Wu2 MSc, FRCPath; Jackie Till2 BSc; Mick Henderson*2 PhD, FRCPath
1Faculty of Medical and Human Science, University of Manchester, Manchester
M13 9PL, UK
2Willink Biochemical Genetics Laboratory, Manchester, Manchester M13 9PL, UK
*Corresponding author
E-mail: Mick.henderson@nhs.net