Rapid Fosfomycin/E. coli NP test: a new technique for the rapid detection of fosfomycin-resistant E. coli isolates
Fosfomycin is a broad-spectrum antibiotic used as empirical treatment for uncomplicated urinary tract infections (UTIs), of which Escherichia coli is the most common cause. To rapidly detect fosfomycin-resistant E. coli isolates and consequently improve patients’ treatment and management, we have developed the Rapid Fosfomycin/E. coli NP test, a rapid, easy-to-perform, specific and sensitive diagnostic test.
by Dr Linda Mueller, Dr Laurent Poirel and Prof. Patrice Nordmann
Introduction
Fosfomycin, a phosphonic acid-derived bactericidal antibiotic discovered in 1969, is now of renewed interest, especially for the treatment of multidrug-resistant (MDR) Gram-negative bacterial infections. This antibiotic is water-soluble and has a low molecular weight, allowing high diffusion at the tissue level [1]. Its features such as broad-spectrum activity, safety and efficacy make fosfomycin as one of the first-line antibiotics used for uncomplicated urinary tract infections (UTIs) treatment [2]. More than 75% of UTIs are due to Escherichia coli [3].
Fosfomycin enters the bacterial cell by the transport proteins GlpT (glycerol-3-phosphate transporter) and UhpT (hexose-6-phosphat:phosphate antiporter); once in the cytosol it binds and inactivates MurA (UDP-N-acetylglucosamine enolpyruvyl transferase), the enzyme involved in the first step of peptidoglycan biosynthesis. Hence, it inhibits bacterial cell wall synthesis [4].
Because of its unique structure and mechanism of action, cross-resistance with fosfomycin and other bacterial agents has not been observed. Fosfomycin as a single agent works well for treating most of UTIs. Additionally, synergistic effects of fosfomycin with several unrelated molecules, such as gentamicin, carbapenems, aztreonam and aminoglycosides, have been observed when treating clinically-relevant MDR Gram-negative bacteria [5].
One of the main concerns with antibiotic resistance in E. coli corresponds to the acquisition of extended-spectrum β-lactamases (ESBL) leading to resistance to expanded-spectrum cephalosporins. ESBL-producing E. coli are mostly community-acquired and may represent 10 to 20% of E. coli isolates in several countries including in the US [6]. Those strains are often co-resistant to several aminoglycosides, to trimethoprim, cotrimoxazole and fluoroquinolones, leaving few therapeutic options available including fosfomycin [7].
Both wild-type susceptible E. coli and ESBL-producing E. coli show an overall high susceptibility rate to fosfomycin (>90%) [8]. However, a Spanish study monitoring fosfomycin resistance during 5 years, showed an increased use of fosfomycin [from 0.122 defined daily dose per 1000 inhabitants per day (DID) in 2004 to 0.191 DID in 2008] and an increased fosfomycin resistance rate in E.coli (from 1.6% to 3.8%) as well as in ESBL-producing E. coli (from 2.2% to 21.7%) [9].
The mechanisms of resistance to fosfomycin described in E. coli are either non-transferable or transferable. The non-transferable and chromosome-encoded resistance involve reduced permeability, resulting from mutations in glpT and uhpT genes, encoding for fosfomycin transporters, and amino acid mutations in the active site of the MurA target. Plasmid-mediated fosfomycin resistance mechanisms in E. coli correspond to production of fosfomycin-inactivating metallo-enzymes (encoded by the fosA genes) [10]. Among the plasmid-borne fosA variants described so far, fosA3 remains the most widespread resistance determinant among both human and animal isolates, those latter being either recovered from pets or livestock [11, 12]. Moreover, a study performed in Taiwan reported the transmission of FosA3-producing E. coli between companion animals and respective owners [13]. Importantly, the fosA3 gene is often identified onto conjugative plasmids along with CTX-M-type ESBL encoding genes, thus leading to acquired resistance to both fosfomycin and broad-spectrum cephalosporins [14, 15]. As fosfomycin is being used as an empiric treatment against UTIs, it was of great interest to develop a rapid test to evaluate the efficacy of this antibiotic.
Rapid Fosfomycin/E. coli NP test
Currently the reference technique recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) to evaluate fosfomycin susceptibility is agar dilution, a fastidious technique requiring 18±2 h to get the results [16]. According to EUCAST, an isolate of E. coli is categorized as susceptible or as resistant when minimum inhibitory concentrations (MICs) are ≤32 and >32 mg/L, respectively.
Alternatively, disk diffusion and gradient strips, although exhibiting some discrepancies with the reference agar dilution method, might be used [17]. To accelerate the process of fosfomycin resistance detection, we have developed the Rapid Fosfomycin/E. coli NP test that allows detection of resistance within 1 h 30 min of fosfomycin-resistant E. coli isolated from culture plates.
This user-friendly technique is based on carbohydrate hydrolysis, detecting bacterial growth of fosfomycin-resistant isolates in the presence of a defined concentration (40 mg/L) of fosfomycin. Of note, fosfomycin-resistant isolates are detected independently of the molecular mechanism of resistance.
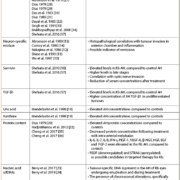
Briefly, the technique includes the preparation of a bacterial suspension (109 CFU/mL; 3–3.5 McFarland) that is poured on a 96-well polystyrene microplate. This culture is made in the Rapid Fosfomycin NP solution supplemented with 25 mg/L glucose-6-phosphate with or without 40 mg/L fosfomycin. The plate is incubated for 1 h 30 min at 35±2 °C and colour changes are detected by visual inspected. Fosfomycin-resistant isolates grow in the presence and absence of fosfomycin, triggering a colour switch from orange to yellow in both wells, a test result which is, therefore, considered as positive (Fig. 1). When dealing with fosfomycin-susceptible isolates, the well supplemented with fosfomycin does not exhibit any bacterial growth and remains orange; the test is, therefore, considered as negative. This test was evaluated with 100 strains including 22 fosfomycin-resistant isolates. It showed a sensitivity and a specificity of 100% and 98.7% respectively.
Conclusion
The Rapid Fosfomycin/E. coli NP test is rapid (1 h 30 min), specific (98.7%) and sensitive (100%). It is easy to perform, cost-effective, and may be used worldwide, regardless of the technical capabilities of the lab. Ongoing work aims to evaluate its performances directly from urine samples, which would represent significant added-value in terms of diagnostic rapidity.
The speed of this test allows a saving of at least 16 h when compared to the traditional agar dilution method. It is a potentially useful clinical test for first-step screening of fosfomycin resistance in E. coli.
Even though a low level of resistance to fosfomycin is currently observed among E. coli, the fact that we usually observe an increased fosfomycin clinical use, meaning an increased selective pressure, argues for a likely increased occurrence of fosfomycin-resistant isolates in the future. Since the principle of this test is based on a rapid culture, it may be used to detect any fosfomycin resistance trait that may be either chromosomally or plasmid-encoded. Fosfomycin is an old antibiotic that is very useful for the treatment of uncomplicated UTIs. On the one hand, even after extensive use for such an indication, the prevalence of resistance remains low, likely due to the fitness cost of the chromosomal mutations needed for acquired resistance, and also as a consequence of a high urinary drug concentration. On the other hand, the worldwide spread of fosfomycin-modifying enzymes should be monitored, as the biological cost of this emerging mechanism of resistance is much lower than that induced by chromosomal mutations [18] and the co-occurrence of fosA-like genes on plasmids with other resistance genes is commonly observed, meaning that co-selection can occur quite frequently.
References
1. Dijkmans AC, Zacarias NVO, Burggraaf J, Mouton JW, Wilms EB, van Nieuwkoop C, et al. Fosfomycin: pharmacological, clinical and future perspectives. Antibiotics (Basel) 2017; 6(4): pii: E24.
2. Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52(5): e103–120.
3. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13(5): 269–284.
4. Castaneda-Garcia A, Blazquez J, Rodriguez-Rojas A. Molecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistance. Antibiotics (Basel) 2013; 2(2): 217–236.
5. Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev 2016; 29(2): 321–347.
6. Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. Contemporary diversity of beta-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent beta-lactamase groups. Antimicrob Agents Chemother 2014; 58(2): 833–838.
7. Wiedemann B, Heisig A, Heisig P. Uncomplicated urinary tract infections and antibiotic resistance-epidemiological and mechanistic aspects. Antibiotics (Basel) 2014; 3(3): 341–352.
8. Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 2010; 10: 4–-50.
9. Oteo J, Orden B, Bautista V, Cuevas O, Arroyo M, Martinez-Ruiz R, et al. CTX-M-15-producing urinary Escherichia coli O25b-ST131-phylogroup B2 has acquired resistance to fosfomycin. J Antimicrob Chemother 2009; 64(4): 712–717.
10. Silver LL. Fosfomycin: mechanism and resistance. Cold Spring Harb Perspect Med 2017; 7(2): pii: a025262.
11. Alrowais H, McElheny CL, Spychala CN, Sastry S, Guo Q, Butt AA, et al. Fosfomycin resistance in Escherichia coli, Pennsylvania, USA. Emerg Infect Dis 2015; 21(11): 2045–2047.
12. Xie M, Lin D, Chen K, Chan EW, Yao W, Chen S. Molecular characterization of Escherichia coli strains isolated from retail meat that harbor blaCTX-M and fosA3 genes. Antimicrob Agents Chemother 2016; 60(4): 2450–2455.
13. Yao H, Wu D, Lei L, Shen Z, Wang Y, Liao K. The detection of fosfomycin resistance genes in Enterobacteriaceae from pets and their owners. Vet Microbiol 2016; 193: 67–71.
14. Benzerara Y, Gallah S, Hommeril B, Genel N, Decre D, Rottman M, et al. Emergence of plasmid-mediated fosfomycin-resistance genes among Escherichia coli isolates, France. Emerg Infect Dis 2017; 23(9): 1564–1567.
15. Yang X, Liu W, Liu Y, Wang J, Lv L, Chen X, et al. F33: A-: B-, IncHI2/ST3, and IncI1/ST71 plasmids drive the dissemination of fosA3 and bla CTX-M-55/-14/-65 in Escherichia coli from chickens in China. Front Microbiol 2014; 5: 688.
16. Performance standards for antimicrobial susceptibility testing, 28th edn. Clinical and Laboratory Standards Institute (CLSI) document M100-S28 2018.
17. Hirsch EB, Raux BR, Zucchi PC, Kim Y, McCoy C, Kirby JE, et al. Activity of fosfomycin and comparison of several susceptibility testing methods against contemporary urine isolates. Int J Antimicrob Agents 2015; 46(6): 642–647.
18. Cattoir V, Guérin F. How is fosfomycin resistance developed in Escherichia coli? Future Microbiol 2018; 13(16): 1693–1696.
The authors
Linda Mueller*1,2 PhD; Laurent Poirel1,2,3 PhD; Patrice Nordmann1,2,3,4 MD, PhD
1Emerging Antibiotic Resistance Unit, Medical and Molecular Microbiology, Faculty of Science and Medicine, University of Fribourg, Fribourg, Switzerland
2Swiss National Reference Center for Emerging Antibiotic Resistance (NARA), University of Fribourg, Fribourg, Switzerland
3INSERM European Unit (IAME, France),University of Fribourg, Fribourg, Switzerland
4University Hospital Center and University of Lausanne, Lausanne, Switzerland
*Corresponding author
E-mail: Linda.mueller@unifr.ch