Novel nephrological markers: anti-PLA2R, anti-THSD7A and uromodulin
Autoantibody diagnostics have in recent years transformed the diagnosis of the rare kidney disease primary membranous nephropathy (MN). The identification of the target antigens M-type phospholipase A2 receptor (PLA2R) and thrombospondin type 1-domain-containing 7A (THSD7A) paved the way for the development of specific immunological assays to detect the corresponding antibodies. Determination of both anti-PLA2R and anti-THSD7A antibodies allows serological diagnosis in 75% to 80% of cases of primary MN. Anti-PLA2R tests are, moreover, an indispensable tool for patient monitoring. A further new biomarker, uromodulin, acts as an indicator of impaired renal function, especially in chronic kidney disease, supplementing established markers such as creatine and cystatin C.
Membranous nephropathy
Membranous nephropathy is an organ-specific autoimmune disease and a major cause of nephrotic syndrome in adults. The disease is characterized by formation of immune complexes in the glomerular basement membrane, resulting in complement-mediated proteinuria and progressive loss of kidney function. 70-80% of cases are of the primary or idiopathic form. The remaining 20-30% of cases are secondary, arising from underlying causes such as malignancy, infection, drug intoxication or another autoimmune disease such as systemic lupus erythematosus. Diagnostic differentiation of primary and secondary forms is crucial due to different treatment regimes. Primary MN is treated with immunosuppressants, while therapy for the secondary form is targeted at the underlying disease. Treatment decisions for primary MN are further complicated by the extreme variability in clinical outcome. Patients can experience spontaneous remission or persistent proteinuria without renal failure, or progress to end-stage renal disease.
Anti-PLA2R antibodies
Autoantibodies against PLA2R are a highly specific marker for primary MN. They occur in around 70% to 75% of patients at time of diagnosis, while they are only very rarely found in patients with secondary MN or in healthy individuals. Their titer, moreover reflects the disease activity and severity. The target antigen, which was identified in 2009, is a type 1 transmembrane glycoprotein which is expressed on the surface of podocytes.
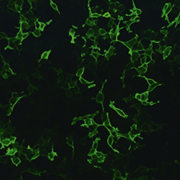
Following the discovery of the target antigen, standardized assays for the determination of anti-PLA2R antibodies in a routine setting were rapidly developed. The recombinant-cell indirect immunofluorescence test (RC-IIFT, Figure 1) utilizes transfected cells expressing full-length PLA2R on the cell surface as the antigenic substrate. The RC-IIFT is a reliable screening test for qualitative detection of anti-PLA2R autoantibodies. Using this assay, anti-PLA2R antibodies were detected with maximum specificity (100%) and a sensitivity of 77% in a cohort of 275 biopsy-proven primary MN patients. In the Anti-PLA2R ELISA, purified recombinant receptor is used as a solid-phase coating of microtitre plates. This assay provides accurate quantification of autoantibody concentrations and is particularly useful for disease monitoring. In a large cohort of clinically well characterized patients, the assay revealed very high sensitivity with respect to the RC-IIFT (96.5%) at a set specificity of 99.9%. The quantitative results of ELISA and RC-IIFT show a good correlation.
Anti-PLA2R is now an established parameter for diagnosing primary MN, differentiating it from secondary MN, assessing the disease status and monitoring responses to therapy [1, 2]. The antibody titre reflects the immunological as opposed to the clinical disease activity, and a change in the antibody titer, either spontaneous or treatment-induced, precedes the corresponding change in proteinuria by weeks or months (Figure 2) [3]. Thus, anti-PLA2R measurements provide a much earlier indicator than proteinuria of patient improvement or deterioration, helping to guide therapy decisions. Complete remission is always preceded by complete antibody depletion.
Anti-PLA2R titres also allow predictions regarding clinical outcome. High antibody titres are associated with a lower chance of spontaneous remission, a longer therapy period to achieve remission, and progression to kidney failure (Table 1) [4]. A low anti-PLA2R antibody titre at baseline, on the other hand, is the most pronounced independent predictor of spontaneous remission [5]. Patients with low anti-PLA2R titres are less likely to require immunosuppressive therapy than those with high titres. Overall, anti-PLA2R assessment is recommended every two months before starting immunosuppressive therapy to avoid unnecessary treatment in patients entering remission, and every month for the first six months of immunosuppression [2].
Anti-PLA2R analysis is also useful for predicting primary MN recurrence after kidney transplantation. Up to 40% of patients relapse after transplantation, and anti-PLA2R positivity is associated with a higher risk of recurrence. In a recent study, pre-transplant anti-PLA2R determination demonstrated a positive predictive value of 100% and a negative predictive value of 91% for a diagnosis of recurrent MN [6]. Further, if anti-PLA2R antibodies are persistently found during the first six months after transplantation, the risk of relapse is particularly high. Antibody determination may therefore be helpful for assessing the necessary and intensity of immunosuppressive therapy following transplantation.
Anti-THSD7A antibodies
Autoantibodies against THSD7A have been recently identified as a further marker in primary MN [7]. Similarly to PLA2R, THSD7A is an N-glycosylated, high-molecular-mass protein expressed on the podocyte membrane. Antibodies against THSD7A occur in around 2.5% to 5% of patients with idiopathic MN. Significantly, they are found predominantly in patients who are negative for anti-PLA2R, suggesting a distinct disease subgroup. Nevertheless, some rare cases with dual positivity for anti-PLA2R and anti-THSD7A have recently been described [8]. No reactivity to THSD7A has been observed in healthy controls or patients with other proteinuric or renal autoimmune diseases.
Anti-THSD7A serves as an additional, complementary marker in primary MN, reducing the diagnostic gap of anti-PLA2R analysis. Moreover, like anti-PLA2R, anti-THSD7A antibody levels also appear to be associated with disease activity. Further studies are currently underway to investigate this link.
Circulating anti-THSD7A antibodies can be determined by RC-IIFT using transfected cells expressing recombinant antigen (Figure 3). Combined testing for anti-PLA2R and anti-THSD7A provides a comprehensive screening for primary MN.
Uromodulin
Uromodulin, also known as Tamm-Horsfall protein, is a glycoprotein which is synthesized exclusively in the kidneys in the ascending limb of the loop of Henle, and subsequently secreted. When renal function is impaired, the uromodulin concentration in the serum or plasma decreases [9]. The concentration exhibits a linear correlation to the estimated glomerular filtration rate (eGFR) (Figure 4). Thus, uromodulin shows inverse kinetics to conventional markers like creatine and cystatin C, which increase with declining kidney function. Moreover, uromodulin concentrations change already in the early stages of chronic kidney disease, when there are few symptoms. Thus, uromodulin measurements enable detection of renal insufficiency in the creatine-blind area in the initial stages of kidney disease. Measurement of uromodulin is also suitable for monitoring kidney vitality during therapy and as a predictive marker after kidney transplantation.
Uromodulin can be measured in the serum or plasma by ELISA based on microplates coated with anti-uromodulin antibodies. The patient uromodulin concentrations are established using a simple cut-off-based interpretation, with a normal value being above 100 ng/ml. External factors such as body weight, nutrition or muscle mass do not need to be factored into the results by additional calculations, as is the case with classic markers. Further, since the uromodulin concentration is measured in serum or plasma, the laborious and error-prone collection of 24-hour urine is not required. This makes it a fast, easy and sensitive supplementary test for the early identification of nephropathies and loss in renal function.
Perspectives
Anti-PLA2R and anti-THSD7A assays are now a mainstay for the diagnosis of primary MN. Due to the high specificity, anti-PLA2R detection may even enable biopsy to be postponed or omitted in elderly patients, persons with poor clinical condition, or patients with life-threatening complications of nephrotic syndrome such as lung emboli. Nevertheless, a proportion of primary MN patients (around 20%) shows negative results for both anti-PLA2R and anti-THSD7A antibodies. This may reflect the disease activity at time of blood sampling (e.g. spontaneous remission) or a misclassification of patients who actually have secondary MN. It is also supposed that some primary MN patients react to other, as yet unidentified antigens. Anti-PLA2R measurements are also playing an increasingly central role in therapy decisions and prognosis, as the relationship between the anti-PLA2R titre and clinical outcome becomes better understood. Current research is directed at further elucidating the complex pathogenesis of primary MN and applying this knowledge to improve therapeutic care.
References
1. Mastroianni-Kirsztajn et al. Frontiers in Immunol. 2015: 6:221
2. Ronco et al. Lancet 2016: 385 (9981): 1983-92
3. Beck et al. Kidney Int. 2010: 77: 765-70
4. Hofstra et al. J. Am. Soc. Nephrol. 2012: 23(10): 1735-43
5. Timmermans et al. Am. J. Nephrol. 2015: 42(1): 70-7
6. Gupta et al. Clin. Transplant. 2016: 30: 461-9
7. Tomas et al. N. Engl. J. Med. 2014: 371(24): 2277-87
8. Larsen et al. Modern Pathol. 2016: 29: 421-6
9. Steubl et al. Medicine 2016: 95(10): e3011
The author
Jacqueline Gosink, PhD
EUROIMMUN AG
Seekamp 31,
23560 Luebeck, Germany
E-mail:j.gosink@euroimmun.de