Point-of-care testing: state-of-the-art and emerging trends
Point-of-care testing (POCT) enables quick test results with minimal manual interference nearer to the site of patient care, which leads to better health outcomes via rapid diagnosis, quick clinical decisions and the early start of treatment. The emerging technologies would further improve POCT by low-cost analysis with increased performance characteristics.
by Dr Sandeep Kumar Vashist and Prof. John H.T. Luong
Existing point-of-care testing (POCT) technologies
A variety of POCT technologies are being used such as POCT analysers, biosensor devices, lab-on-chips (LOC), test strips, and lateral flow assay (LFA) cartridges. Such cost-effective technologies offer rapid analysis in just a few minutes using minimal sample volumes. As well as at a patient’s bedside, POCT have been employed in the operating theatre, emergency department and critical care/maternity unit. Other deployments include nursing homes, physician’s office, prison, emergency vehicles, etc. Local pharmacies have adopted the POCT technology to provide a one-stop service for glucose, cholesterol, pregnancy, etc.
The POCT analysers are standard bench-top devices that can determine a broad range of analytes, based on spectrophotometry, reflectometry, immunoassay, turbidimetry, potentiometry/amperometry, oximetry and hematological particle counting. The target analytes are small metabolites, enzymes, drugs-of-abuse, inflammation biomarkers, heart and kidney injury biomarkers, infectious agents, humoral and cellular coagulation markers, hematological parameters, etc.
The biosensor-based devices, notably the blood glucose meters, are the most common POCT technology, which have been widely used for the detection of glucose. Different LOC platforms are being used in various POCT technologies. They are fully automated platforms that integrate all microfluidics-based bioanalytical steps such as sample treatment, separation, biomolecular detection, washing, signal detection, and data processing, storage, and transmission. The signal detection in most LOC platforms employs optical readout. The technologies and materials used for the production of LOC platforms have been fully characterized and standardized.
Test strips are another prominent POCT technology for detecting different analytes in a patient’s blood or urine sample. They are easy to use and easy to read, leading to immediate on-the-spot analysis. The test strip comprises a solid support onto which porous matrices with dried assay reagents are integrated. The reaction starts as the biorecognition element present on the test strip detects the analyte, which leads to a visual change in colour on the test strip. The signal is read by inserting the test strip into a reader device.
LFA, one of the most widely used POCT technologies, is based on an immunochromatography format where the assay reagents are stored in dried form on various porous materials. Once the sample (urine or diluted blood) is dispensed at the designated area of the LFA cartridge, the sample flows in the lateral direction by capillary forces. It first interacts with the capture antibodies spotted at the reaction area leading to the formation of the immune complex, which is followed by binding to the detection antibodies immobilized at another area of the cartridge, thereby resulting in the formation of the sandwich immune complex. This leads to a visible colour change in the test and control lines, which facilitates rapid qualitative or semi-quantitative analysis. The POC pregnancy testing is done exclusively by LFA.
The multiplex analysis using DNA and protein microarrays is also being intensively investigated although the technology has not yet been commercialized for clinical POCT. It is envisaged that this upcoming technology would be fully automated, which would involve advanced microfluidics and the signal readout by electrochemical, chemiluminescence, fluorescence or evanescent wave techniques.
Emerging POCT technologies
Various POCT technologies have emerged during the last decade, which have tremendous potential for next-generation healthcare monitoring and management [1]. In 2011, the estimated total POCT market was US$15 billion and projected to reach US$18 billion by 2016. Of the total POCT market in 2011, 55% of it was in the US market, followed by 30% in Europe and 12% in Asia [2].
Cellphone (CP)-based devices
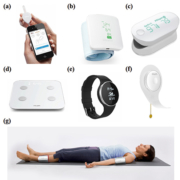
The most prospective emerging technology is CP-based devices. CPs have become ubiquitous with more than 7 billion global users that account for more than 95% of the world’s population. Moreover, about 70% of the CP users reside in the developing countries, where there is an imminent need for mobile healthcare (mH). The current generation of CPs are cost-effective and equipped with all the desired advanced features that facilitate personalized mH monitoring and management. The spatiotemporal tagging of the data by CP enables the real-time active response to epidemics and emergency situations. Various FDA approved and CE certified CP-based personalized healthcare devices have already been commercialized for the monitoring of basic physiological parameters such as blood glucose, blood pressure, pulse rate, blood oxygen saturation, body weight, body analysis parameters, electrocardiogram, physical activity, sleep and cardiac parameters (Fig. 1) [3]. Most of such commercial CP-based devices are developed by iHealth Labs, France. Similarly, CP-based technologies have been prepared for many POCT applications [4]. Cellmic, USA has developed a compact CP-based rapid-diagnostic-test reader for the readout of colorimetric and fluorometric LFA (Fig. 2). Of notice is the conversion of the CP into a compact and lightweight computational microscope for bright field, fluorescence, darkfield, transmission and polarized microscopy modes. Moreover, a CP-based flow cytometer based on optofluidic fluorescent imaging enabled the screening of pathogens in whole blood or water samples [5]. Another similar endeavour is the development of smartphone-based spectrophotometers for the detection of absorbance, fluorescent or chemiluminescent signals [6]. Various CP-based colorimetric readers [7], electrochemical sensing platform [8], angle-resolved surface plasmon resonance (SPR) system [9], and multiplex assays have also been developed [10].
Paper-based diagnostics
Considering simplicity and cost-effectiveness, paper-based diagnostics (PBD) are available in various formats: LFA, dipstick and microfluidic paper-based analytical devices (µPADs) [11, 12]. LFA are widely used in home pregnancy test strips to detect human chorionic gonadotropin in urine. It employs the dispensing of the sample onto the sample pad of the LFA test strip (fabricated from a nitrocellulose membrane). The sample flows laterally over a conjugate pad due to the capillary action provided by the absorbent pad, which leads to the binding of the analyte to conjugate particles (gold nanoparticles and upconversion nanoparticles). The signal detection can be visual, colorimetric, electrochemical, photoelectrochemical, chemiluminescent and electrochemiluminescent. The colorimetric PBD provide qualitative analysis by comparing the colour against a predetermined score chart. But the colour intensity can also be quantified using cameras, scanners, commercial test strip readers and hand-held colorimeters. A CP-based rapid-diagnostic-test reader is the most recent development that enables precise determination of colour intensity [13].
Paper can be patterned to fabricate two-dimensional (2D) or three-dimensional (3D) µPADs. The 3D µPADs, formed by stacking layers of the 2D paper, are ideal for multiplexing. The microfluidic paper-based electrochemical devices (µPEDs) can be fabricated by printing electrodes on paper.
The sensitivity of PBDs can be increased by employing enzymes and nanomaterials based signal enhancement strategies, which increases the costs and assay duration, and decreases the shelf-life. However, PBDs suffer from poor reproducibility, non-uniformity and variable accuracy on µPADs due to the passive capillary transport in paper substrates.
Lab-on-a-chip platforms
Various LOC platforms, such as the most widely used blood glucose testing strips, have been made for POCT. These platforms enable fully automated analysis by integrating all process steps in the operational procedure. The Piccolo Xpress™ whole blood chemistry analyser, developed by Abaxis Inc, is a prospective LOC-based POCT device that performs 14 tests on a single reagent LabDisk (8 cm diameter, barcoded).
Rapid assay formats
A prospective assay format is Optimiser™ ELISA [14] by Siloam Biosciences Inc, which employs a novel microfluidic microtiter plate. It detects an analyte in just a few minutes using minimal reagent volumes and least number of steps. Other prospective formats are the wash-free AlphaLISA® by Perkin Elmer, wash-free electrochemiluminescent ELISA by Meso Scale Diagnostics LLC, rapid one-step kinetics-based formats [15], CP-based easy immunoassay platforms [16], and COBAS® Lab-in-a-Tube (LIAT) system by Roche Diagnostics.
Prolonged reagent storage strategies
The most prospective prolonged reagent storage strategies are the use of polysaccharides and saccharides (such as pullulan and trehalose), sugar alcohols, stabilizers, freeze drying, lyophilization, and reagent pouches. Use of POCT in remote areas, particularly in developing countries might be problematic due to inconsistent electrical power, lighting, and refrigeration.
Conclusions
The next decade will witness the revolutionary breakthroughs in POCT that will drastically cut down the costs and lead to considerably improved analysis with increased bioanalytical performance and capabilities. Multi-channel, high-throughput instruments are expected to expand the new concept of POCT, and there is an obvious need for the combination of POCT technology and communication technology. Non-invasive blood glucose monitoring remains the most important market for POCT, followed by coagulation, blood gas, chemistry, hematology, urinalysis, and cardiac. Also, molecular POCT technology has matured and began to move toward commercialization.
Future trends for POCT will rise due to new emerging technologies to make them well suited for low-resource or remote areas. POCT vendors increasingly offer more available types of tests with improved accuracy and minimal turnaround times. Thus, POCT will be more widely accepted as an addition to the current method of managing patients. Considering its limited test menus, clinicians still have to wait for other sophisticated laboratory tests before the action can be taken for treatment or further testing [17]. POCT evolves continuously and becomes an ‘extension’ of laboratory services, not a replacement of routine core laboratory testing.
References
1. Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JHT. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015; 33(11): 692–705.
2. Scientia Advisors. The point-of-care diagnostics market. 2013, Cambridge, MA, USA. http://www.businesswire.com/news/home/20100915005199/en/Scientia-Advisors-Projects-Sustainable-Growth-Point-of-Care
3. Vashist S, Schneider E, Luong JHT. Commercial smartphone-based devices and smart applications for personalized healthcare monitoring and management. Diagnostics 2014; 4(3): 104–128.
4. Vashist SK, Mudanyali O, Schneider EM, Zengerle R, Ozcan A. Cellphone-based devices for bioanalytical sciences. Anal Bioanal Chem. 2014; 406(14): 3263–3277.
5. Zhu H, Mavandadi S, Coskun AF, Yaglidere O, Ozcan A. Optofluidic fluorescent imaging cytometry on a cell phone. Anal Chem. 2011; 83(17): 6641–6647.
6. Yu H, Tan Y, Cunningham BT. Smartphone fluorescence spectroscopy. Anal Chem. 2014; 86(17): 8805–8813.
7. Vashist SK, van Oordt T, Schneider EM, Zengerle R, von Stetten F, Luong JHT. A smartphone-based colorimetric reader for bioanalytical applications using the screen-based bottom illumination provided by gadgets. Biosens Bioelectron. 2015; 67: 248–255.
8. Lillehoj PB, Huang MC, Truong N, Ho CM. Rapid electrochemical detection on a mobile phone. Lab Chip. 2013; 13(15): 2950–2955.
9. Preechaburana P, Gonzalez MC, Suska A, Filippini D. Surface plasmon resonance chemical sensing on cell phones. Angew Chem Int Ed Engl. 2012; 51(46): 11585–11588.
10. Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, Olowookere OO, Cadinu P, Meng F, Chee NH, et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci Transl Med. 2015; 7(273): 273re1.
11. Mao X, Huang TJ. Microfluidic diagnostics for the developing world. Lab Chip 2012; 12(8): 1412–1416.
12. Li X, Ballerini DR, Shen W. A perspective on paper-based microfluidics: current status and future trends. Biomicrofluidics 2012; 6(1): 11301–11301-13.
13. Mudanyali O, Dimitrov S, Sikora U, Padmanabhan S, Navruz I, Ozcan A. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip 2012; 12(15): 2678–2686.
14. Kai J, Puntambekar A, Santiago N, Lee SH, Sehy DW, Moore V, Han J, Ahn CH. A novel microfluidic microplate as the next generation assay platform for enzyme linked immunoassays (ELISA). Lab Chip 2012; 12(21): 4257–4262.
15. Vashist SK, Czilwik G, van Oordt T, von Stetten F, Zengerle R, Marion Schneider E, Luong JHT. One-step kinetics-based immunoassay for the highly sensitive detection of C-reactive protein in less than 30min. Anal Biochem. 2014; 456: 32–37.
16. Vashist SK, Czilwik G, Venkatesh AG. Elisa system and related methods. WIPO Patent Pub No WO/2014/198836; 2014.
17. Boonlert W, Lolekha PH, Kost GJ, Lolekha S. Comparison of the performance of point-of-care and device analyzers to hospital laboratory instruments. Point of Care 2003; 2(3): 172–178.
The authors
Sandeep Kumar Vashist*1 PhD, John H.T. Luong2 PhD
1Vallo Med Health Care GmbH, Castrop-Rauxel, Germany
2Innovative Chromatography Group, Irish Separation Science Cluster (ISSC), Department of Chemistry and Analytical, Biological Chemistry Research Facility (ABCRF), University College Cork, Cork, Ireland
*Corresponding author
E-mail: sandeep.vashist@vallomed.com