Competitive PCR-high resolution melting analysis: an improved approach to assess BRCA status in hereditary breast and ovarian cancer patients
The identification of BRCA pathogenic variants (PVs) is the major concern for the genetic counselling in families with a high risk of breast (BC) and ovarian cancer (OC). BRCA1 (breast cancer early onset 1) and BRCA2 (breast cancer early onset 2) are the two major susceptibility genes in BC/OC, conferring a lifetime risk up to 87% for BC and up to 44% for OC. BRCA mutations have been found in 4–14% of all OC, with a higher occurrence of about 22% in the high-grade serous OC [1]. The clinical relevance of the identification of BRCA PV carriers concerns many aspects of a patient’s evaluation. The first relevant implication is the assessment of the lifetime cancer risk. Additionally, BRCA testing has a relevant impact on the therapeutic approach and on the treatment outcomes owing to the possibility of selecting patients for biomarker-directed therapy based on the mutational status [2]. BRCA-positive patients with OC, particularly, respond well to platinum-based chemotherapy, especially in the high-grade serous OC subtype, and tend to retain platinum-sensitivity for longer than those without BRCA PVs. Additionally, the treatment with poly (ADP-ribose) polymerase (PARP) inhibitor (e.g. olaparib) was approved as a target therapy in patients with both germline and somatic BRCA PVs. PARP inhibitor therapy is able to improve progression-free survival in response to a recent platinum-based chemotherapy [3]. To date, licensed PARP inhibitor is part of the standard care and, consequently, BRCA evaluation is considered a routine investigation tool, useful before treatment management. With respect to these benefits, BRCA testing should be offered to all patients with OC on the basis of histological subtype, regardless of age, or family and personal history of malignancy. This issue causes an increase of the demand for BRCA testing with a strong challenge into the diagnostic laboratories committed in fulfilling the need of an efficient and rapid molecular evaluation [4].
The challenge of BRCA testing
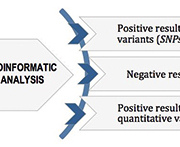
To date 1700 PVs in BRCA1 and 1900 PVs in BRCA2 have been reported. Most of them are single nucleotide polymorphisms (SNPs) or small insertion-deletion mutations (indels), with a significant impact on the structure and function of the protein. Also large genomic rearrangements (LGRs), consisting mainly in large deletions or duplications, represent an important part of BRCA molecular lesions. To date, a total of 98 different BRCA LGRs have been reported, 81 in BRCA1 and 17 in BRCA2 [5] with a prevalence that varies considerably. Interestingly, deletion of BRCA1 exon 1a-2 is reported in several populations worldwide and is considered a recurrent BRCA LGRs in BC/OC patients [6]. Owing to the broad complexity in the mutational landscape of BRCA genes, comprehensive screening including the efficient assessment of both qualitative (SNPs, indels) and quantitative (LGRs) alterations is mandatory (Fig. 1). Diagnostic laboratories are adopting next-generation sequencing (NGS) technology for BRCA testing, which offers the potential of fast, cost-efficient and comprehensive sequencing. By choosing NGS technology, many considerations should be made, such as the selection of an NGS platform, including the enrichment methods, the sequencing chemistries, the analytical procedures and the variant calling for both germline and somatic PVs [2]. NGS is highly recommended as the reference sequencing method for BRCA testing because of the size of coding region and the method’s sensitivity in tumour sample evaluation. In fact, Sanger sequencing is not suitable for the analysis of somatic mutations, especially in samples where the percentage of tumour cells is under 50%, and it requires also a large amount of starting DNA [4]. Several methods are commonly used for LGR analysis, including multiplex ligation-dependent probe amplification (MLPA) and multiplex amplicon quantification (MAQ). However, these approaches are expensive and time-consuming, and consequently these are not always suitable for all laboratories. In this case, LGR evaluation of BRCA genes represents a bottleneck in terms of time and costs. In this context, the great benefit of the NGS approach is the opportunity to obtain both qualitative and quantitative information from the same sequencing data by using tailored bioinformatics algorithms [7]. Only a positive bioinformatics result needs to be confirmed using an alternative conventional method. In order to optimize our routine diagnostic procedures for BRCA testing, we recently developed a new molecular approach called competitive PCR-high resolution melting analysis (cPCR-HRMA) [8], as an alternative method for LGR identification in BRCA genes. HRMA is a simple and robust closed-tube method commonly used for diagnostics, forensic and research purposes. This method consists of a PCR amplification performed in the presence of saturating binding dyes followed by a melting reaction. Specifically, the incremental increase of the reaction temperature causes the denaturation of double-stranded DNA with the concomitant release of intercalated dye and a decrease of fluorescence signal. The specific sequence of the analysed amplicon, primarily relating to the GC content and the length, determines the melting behaviour observed in a fluorescence signal versus temperature plot. Additionally, the melting temperature (Tm) may be calculated as the derivative of the melting curve. The shape of the curves and the specific Tm value obtained in the output plots is used for the genotyping. The advantages of this technique include rapid turn-around times and a closed-system environment that decrease the risk of laboratory contamination [9].
cPCR-HRMA for LGR evaluation
HRMA technology is typically applied to detect a single substitution, as well as small indels variants [6, 10]. The new cPCR-HRMA represents an optimized HRMA method that allows an efficient evaluation of BRCA1 copy number variation (CNV) by relying on the melting behaviour of target BRCA amplicon compared to a reference amplicon in the same HRMA reaction. In particular, specific albumin sequences were chosen as unchanged CNV references and analysed by coupling them with specific BRCA1 exons in a duplex PCR assay preceding the melting analyses. The landmarks of this new HRMA rely on the primers and the amplification protocol design. First of all, primer pairs for the simultaneous amplification of target and reference sequences are selected in order to produce paired amplicons with comparable lengths (similar amplification efficiencies) and different melting temperatures (no overlap between amplicons melting peaks). Furthermore, the primer concentration used for both target and reference amplification was set in order to produce comparable PCR performance between the two amplicon types and to obtain melting profiles more suggestive of CNV. In addition, the PCR thermal cycling was carried on until the exponential phase, in which the amplification performance reflects the CNV status of the target region. These optimized features lead to melting profiles specifically tailored for CNV investigations allowing a rapid detection of samples affected by a change in copy number. Genotype association was assessed by direct interpretation of melting profiles, as shown in Figure 2: samples with similar profiles were clustered into the same genotype group and CNV positive samples showed a typical melting profile with a detectable shape comparing to the wild type. In addition to the qualitative evaluation, we provide also a semi-quantitative analysis of melting behaviour with the calculation of the fluorescence peak height ratio (R) of target the amplicon (BRCA1) to the reference amplicon (albumin), according to the formula:
The mean and the standard deviation of the R values calculated in a consistent number of control CNV samples allowed the identification of the reference range for the R parameter: WT sample (mean±2SD; 2 copies), deletion (≤mean−2SD; n copies) and duplication (≥mean+2SD; 3n copies). The R value calculated in each analysed sample is normalized with the average of the ratios calculated in the WT sample group, obtaining the normalized fluorescence peak height ratio (Rn). The latter is compared to the reference range in order to obtain the copy number interpretation. Taken together, the qualitative and the semi-quantitative evaluations of the cPCR-HRMA assay allow the correct identification of copy number status in BRCA gene, resulting as a rapid and alternative method for the analysis of LGRs. Advantages of cPCR-HRMA are the ease and fast handling of samples. Furthermore, this application needs the same reagents and equipment for standard HRMA protocols commonly used in many laboratories routines. By introducing this efficient alternative method, our first aim was the optimization of the BRCA workflow, promoting a more rational use of confirmatory testing, such as MLPA and MAQ. Finally, we are confident that a general implementation of BRCA testing is now necessary as an emerging challenge. After a complete genetic counselling and a multidisciplinary activity that involves geneticists, oncologist and all other professionals, the patient should be directed to specialized laboratories. The complexity of the potential BRCA mutations, coupled with their clinical relevance, leads to the mandatory adoption of a comprehensive molecular workflow for BRCA analysis that must be characterized by a low wait-time and efficient clinical reporting in order to guarantee a useful medical application.
References
1. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003; 72(5): 1117–1130.
2. Capoluongo E, Ellison G, López-Guerreroc JA, et al. Guidance statement on BRCA1/2 tumor testing in ovarian cancer patients. Semin Oncol 2017; 44(3): 187–197.
3. George A, Kaye S, Banerjee S. Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat Rev Clin Oncol 2017; 14(5): 284–296.
4. Capoluongo E, Scambia G, Nabholtz JM. Main implications related to the switch to BRCA1/2 tumor testing in ovarian cancer patients: a proposal of a consensus. Oncotarget 2018; 9(28): 19463–19468.
5. Sluiter MD, van Rensburg EJ. Large genomic rearrangements of the BRCA1 and BRCA2 genes: review of the literature and report of a novel BRCA1 mutation. Breast Cancer Res Treat 2011; 125: 325–349.
6. Mazoyer S. Genomic rearrangements in the BRCA1 and BRCA2 genes. Hum Mutat 2005; 25(5): 415–422.
7. Scaglione GL, Concolino P, De Bonis M, et al. A whole germline BRCA2 gene deletion: how to learn from CNV in silico analysis. Int J Mol Sci 2018; 19(4): pii: E961.
8. Minucci A, De Paolis E, Concolino P, et al. Competitive PCR-high resolution melting analysis (C-PCR-HRMA) for large genomic rearrangements (LGRs) detection: a new approach to assess quantitative status of BRCA1 gene in a reference laboratory. Clin Chim Acta 2017; 470: 83–92.
9. Erali M, Voelkerding KV, Wittwer CT. High resolution melting applications for clinical laboratory medicine. Exp Mol Pathol 2008; 85(1): 50–58.
10. De Paolis E, Minucci A, De Bonis M, et al. A rapid screening of a recurrent CYP24A1 pathogenic variant opens the way to molecular testing for idiopathic infantile hypercalcemia (IIH). Clin Chim Acta. (2018) Mar 21; 482: 8–13.
The authors
Elisa De Paolis MSc, Angelo Minucci PhD, Giovanni Luca Scaglione PhD, Maria De Bonis MSc, Ettore Capoluongo* PhD
Catholic University of The Sacred Heart, Rome, Italy
*Corresponding author
E-mail: ettoredomenico.capoluongo@policlinicogemelli.it