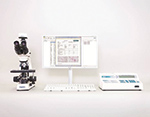
Digital system for sperm analysis
Vision Sperm is an integrated system for conducting sperm quality analysis and managing microscopy samples, patient data and analysis results. The system makes it possible to store and examine digital sperm samples and analysis results. Accurate and objective analysis supported by report documentation and specialized soft ware provides greater assurance in analysis results. Working with the Vision Sperm system does not lead to fatigue and red eyes and reduces the load on neck and hand muscles that accompanies routine microscopy. By working with the system, doctors and laboratory professionals improve their expertise every day, thanks to the use of atlas, review of images and comments as well as discussion with colleagues and experts. Information exchange makes up for a lack of specialists. Accurate diagnosis and an appropriate strategy for further examination will ensure correct treatment of the patient.
Read more