CRP point-of-care test
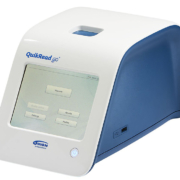
QuikRead go system is an extremely fast point-of-care test system for measuring C-reactive protein (CRP) in whole blood samples: CRP results are available in two minutes. The system simplifies the daily routines of healthcare professionals by bringing reliability and speed to everyday working processes. The intuitive user interface with a large touch screen and a variety of user interface language options add to the easiness of use. The CRP test can be performed near the patient and the CRP result is immediately available to support the diagnosis. The test selection includes CRP, CRP+Hb, hsCRP+Hb, iFOBT and Strep A tests. This year two new products, wrCRP and wrCRP+Hb, will be launched in the product portfolio.