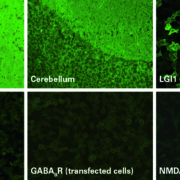
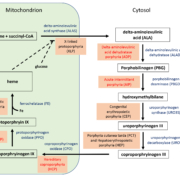
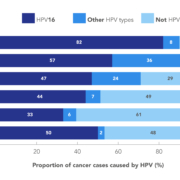
Digitization of health
The development of appropriate technical instrumentation for both sample and data analysis in recent years has driven progress in the field of metabolomics. CLI chatted to Dr Ali Tinazli (CEO at lifespin GmbH) to find out more about how this process of ‘digitizing health’ is developing and what it will mean for us as inidviduals.