Urine microscopy analyser
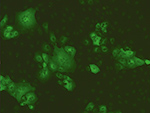
The UriSed mini is a professional semi-automated urine microscopy analyser producing whole fields of view microscopic images of urine sediment, and automatically classifying and counting urine sediment particles in the images. UriSed mini utilizes the traditional gold standard method while eliminating the most time-consuming and operator-dependent procedures in laboratories performing manual microscopy. In addition it can also serve as a backup instrument of automated sediment analysers. Main features include automatic identification of urine particles by the Auto Image Evaluation Module (AIEM); total measurement cycle of less than 1 minute; cost-effective operation without liquid reagents or calibrators. Easy to operate and requiring minimal training, the analyser is a highly effective tool in a wide range of medical and clinical settings.