Alinity h-series hematology systems
Simplicity built in. Streamlined for your laboratory.
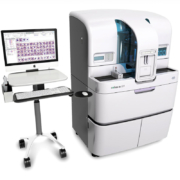
The Alinity h-series of systems integrates hematology workflow, from high-throughput Complete Blood Count (CBC) analysis to automated slide making and staining. With a bi-directional, internal conveyor and a throughput of 125 CBCs per m2, it delivers high performance in one of the most compact footprints available.
The system is composed of two Alinity hq modules and an Alinity hs module in a hq-hs-hq configuration. The Alinity hq analyzer delivers a CBC with an expanded 6-part White Blood Cell (WBC) differential that includes routine and several advanced parameters using Advanced MAPSS (Multi Angle Polarized Scatter Separation) technology. With the Alinity hs slide maker stainer and the integrated robotic sample management, the Alinity h-series of systems delivers unprecedented uniformity, flexibility, operational productivity and confidence to your customers.
Achieve integration that is built on user-focused design
With an emphasis on addressing needs articulated by you, the Alinity h-series of solutions offer an intuitive and uniform experience, aligned with other Alinity solutions. This commonality in user experience across multiple laboratory disciplines, allows for efficiencies in training, operation, and utilization of staff.
Deliver performance that comes from proven expertise in system and assay design
The Alinity h-series was designed from the ground-up with innovative technology and design elements that will address your need of managing a changing workload with existing resources. Alinity hq and Alinity hs have been designed to help you improve your laboratory’s operational productivity by automating routine processes, allowing your staff to focus on critical tasks.
Not commercially available in all countries, including the USA.
For invitro diagnostics use only.
ADD-00062367
Read more