Glucose meter offers wireless connectivity
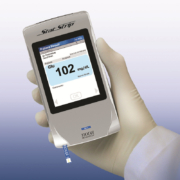
Nova Biomedical’s StatStrip Glucose Hospital Meter System now offers bidirectional wireless connectivity to the hospital’s HIS or LIS with complete security to protect patient data. The wireless connectivity can transmit patient results directly from the patient bedside, alleviating the need to bring the meter to a fixed location for meter docking and data transmission. Wireless connectivity saves time for the caregiver and allows for faster charting of results and clinical decision making to improve patient care. StatStrip Glucose’s dual-band wireless connectivity provides complete security and encryption to ensure that patient data remains uncompromised. Nova now offers a full range of StatStrip Glucose wireless connectivity capabilities, including wireless meters, wireless carrying cases, and wireless docking stations. All of the wireless devices use industry standard POCT1-A2 data format and are compatible with a choice of middleware partners. StatStrip Glucose is the only glucose meter cleared by the U.S. Food and Drug Administration (FDA) for use with all patients, in all healthcare settings, including critical care. The device received this clearance in 2014 after an extensive, nearly four-year study conducted at five major university medical centres, which included 1,698 critically ill patients with over 257 clinical conditions. Over 8,000 medications were investigated for potential interference to StatStrip Glucose measurement. The device demonstrated excellent agreement compared to central laboratory reference methods and no clinical interferences were found. In addition to the study submitted to the FDA, 138 other independent studies over the last eight years—including 53 critical care studies—have found no clinically significant interferences for StatStrip Glucose.